Abstract
Anthropogenic perturbations and climate change are severely threatening habitats of the global ocean, especially in the Arctic region, which is affected faster than any other ecosystem. Despite its importance and prevailing threats, knowledge on changes in its micro- and nanoplanktonic diversity is still highly limited. Here, we look back almost two decades (May 1–26, 2002) in order to expand the limited but necessary baseline for comparative field observations. Using light microscopy, a total of 196 species (taxa) were observed in 46 stations across 9 transects in the Greenland Sea. Although the number of observed species per sample ranged from 12 to 68, the diversity as effective species numbers (based on Shannon index) varied from 1.0 to 8.8, leaving about 88% as rare species, which is an important factor for the resilience of an ecosystem. Interestingly, the station with the overall highest species number had among the lowest effective species numbers. During the field survey, both number of rare species and species diversity increased with decreasing latitude. In the southern part of the examined region, we observed indications of an under-ice bloom with a chlorophyll a value of 9.9 μg l−1 together with a nitrate concentration < 0.1 μM. Further, we recorded non-native species including the Pacific diatom Neodenticula seminae and the fish-kill associated diatom Leptocylindrus minimus. Our comprehensive dataset of micro- and nanoplanktonic diversity can be used for comparisons with more recent observations and continuous monitoring of this vulnerable environment—to learn from the past when looking towards the future.
Similar content being viewed by others
Introduction
Globally, ecosystems undergo dramatic changes due to climate change and anthropogenic perturbations. Since the Arctic ecosystem (here defined as north of 70°) is experiencing climate change at a much higher speed compared with the rest of the globe, it has been designated as one of the most vulnerable (IPCC 2018) and, thus, might exemplify future situations of other environments. The most apparent transformation is the Arctic ice system, becoming seasonal rather than perennial within the next 50 years due to elevated air and surface water temperatures (Wassmann and Reigstad 2011). With less ice, the growth season of phytoplankton may be prolonged (Lebrun et al. 2019; Renaut et al. 2018). Although a rapidly changing environment is expected for the phytoplankton communities in the Arctic region, knowledge on how this will affect their composition and diversity is still very limited.
High diversity among and within species is important in all ecosystems, elevating resilience to environmental change (Hooper et al. 2012). Due to human perturbations at the planetary scale, biodiversity is currently suffering from an increased risk of great losses (Kannan and James 2009; Steffen et al. 2015). On the other hand, a biodiversity change can be as devastating, resulting in potential effects on ecosystem services (Dornelas et al. 2014). Thereby studies need to include metrics estimating species richness, together with changes in species composition (Hillebrand et al. 2018). Combination of species numbers and diversity metrics enables quantification of the rare biosphere in, for example, a phytoplankton community. The importance of recruitment from the rare biosphere has been demonstrated for bacteria as a response to environmental changes (Sjöstedt et al. 2012) but may also be important for resilience in microplanktonic communities in the Arctic region. A decreased diversity can result in a less resilient ecosystem, i.e., with only few species in each functional group resulting in a community at a high risk upon species loss (Snoeijs-Lejonmalm 2017).
Microplanktonic communities can, for example, be disturbed by the introduction of non-native species, where human activities facilitate the global movement at a higher rate than would occur naturally (Molnar et al. 2008). Although the Arctic region was long considered a low-risk environment due to harsh conditions and limited access, elevated temperatures and ice retreat have now opened up for shipping and tourism, facilitating the introduction of non-native, potentially invasive species (Chan et al. 2019; Melia et al. 2016; Ricciardi et al. 2017). Near-surface transport is expected to increase with elevated temperature and, thus, affect the natural spread of microplankton species (Arrigo and van Dijken 2004; Jones et al. 2003; Poulin et al. 2010), as reported for the North Sea (Nehring 1998). Newly introduced species have the potential to affect local food chains through, for example, production of harmful substances or by altering the nutritional value for grazers.
A recent comprehensive study compiling invasive species events in the Arctic only briefly addressed microalgae (Chan et al. 2019), since knowledge on changes in these communities is limited (Niemi et al. 2011; Assmy et al. 2017) and are often conducted using sequencing techniques (Kilias et al. 2014; Karlusich et al. 2020). Although species lists “on the surface of the sea between Europe and Greenland as well as from the Davis Straight” have been reported as far back as 1873 (Cleve 1873), they were dominated by diatoms due to the ease of preservation compared with flagellates and other phytoplankton. Species lists (published in English) 5–10 years before our study include Gradinger and Baumann (1991), Samtleben et al. (1995), Bauerfeind et al. (1997), Booth and Smith (1997), von Quillfeldt (1997), and Kohly (1998). This list can be expanded to seas and basins on similar latitudes such as the Laptev Sea (Tuschling et al. 2000), the Barents Sea (Ratkova and Wassman 2002; Sergeeva et al. 2018), Baffin Bay (Lafond et al. 2019), and waters around Svalbard (Owrid et al. 2000).
Since both changes in species composition and introductions of non-native species can drastically affect the Arctic ecosystem food web, a species diversity baseline to detect early ecosystem changes is highly needed. Thus, by defining a baseline of species diversity and community composition we may monitor ecosystem changes for management purposes. The present study looks back almost two decades with the aims to (a) assess micro- and nanoplankton species diversity in the Greenland Sea along the ice-covered coastline and (b) to reveal potential northward spread of species across a latitudinal gradient. Hence, this study may work as a template for future monitoring surveys.
Material and methods
Site description
The expedition with the Swedish icebreaker Oden covered the East Greenland Current from north of Fram Strait to south of Denmark Strait as a part of the Arctic Ocean 2002 program (AO-02, see e.g., Nilsson et al. 2008 and Rudels et al. 2005 for further details on the physicochemical parameters along the transects). More specifically, the expedition followed the east side of Greenland (82° 14’ N to 64° 46’ N) and was conducted between April and June in 2002. The Fram Strait north of Svalbard and Greenland was covered with sea ice at the time of sampling (indicated in Fig. 1). The micro- and nanoplankton sampling was conducted between the 1st and 26th of May along 9 transects with a total of 46 sampling stations (Fig. 1 and Table 1). All sampling transects started from the sea and continued towards the ice-covered coastline of east Greenland.
CTD data for salinity and temperature were obtained using a CTD SBE 911 + instrument (see Rudels et al. 2005). Salinity ranged between 32 and 35 across stations and was similar between surface (~ 2 m) and deep waters (10–50 m), suggesting a well-mixed upper water mass. The lowest salinities were observed close to the ice edge (Table 1). Air temperature during the expedition ranged from − 30° C in the north (83° N) to 0° C between Greenland and Iceland (65° N). Water temperatures showed larger variation between stations, as compared with between surface and deep waters (− 1.82 to 0.22 °C, stations 10–76, and − 1.72 to 6.61 °C in stations 80–95, Table 1). Higher temperatures were observed furthest away from the ice and towards the south, coinciding with stations sampled later in the season.
Sea water sampling, inorganic nutrients, and light intensity
Prior to each water sampling, fluorescence was measured to estimate the depth of the deep chlorophyll a maximum (DCM), using a handheld “mini CTD” of ADM model, equipped with a fluorometer. Seawater samples were collected from the SeaBird Carousel rosette sampler equipped with 12 L Niskin bottles, or from Go-Flo sampling bottles. Generally, seawater was sampled from the surface (1–3 m depth depending on weather conditions) hereafter called “surface” and from DCM (10–50 m), hereafter referred to as “deep” sampling. If there was no distinct DCM due to a well-mixed water column or low chlorophyll a (Chl a) values, seawater was sampled from 20-m depth (sampling depths for each station and parameter can be found in Table S1). Inorganic nutrient concentrations (nitrate, nitrite, phosphate, and silicate) were extracted from the expedition database. In short, inorganic nutrients were analyzed on an auto-analyzer according to the WOCE protocol (Gordon et al. 1993). Light intensity (PAR 400–700 nm) in air was occasionally measured using a light meter (International Light 1400 A) equipped with a PAR sensor (IL SEL033).
Micro- and nanoplankton community composition and diversity
From each depth (Table S1), 2 L of seawater was gently filtered onto each of two 2-μm Nucleopore polycarbonate filters (1 L each), one put in acidic and one in basic Lugol’s solution for later qualitative and quantitative analyses using the Utermöhl technique (Utermöhl 1958). All cells from each of the filters were rinsed into a concentrated sample that was left to settle for counting (transects, views) in the sedimentation chamber. Large, less abundant cells were counted in low magnification and small, abundant cells in higher magnification, in order to include as many cells as possible. Fewer transects were counted for the common organisms and the whole bottom area of the chamber (equal to 1-L concentrated sample) for the less abundant ones. On-board documentation (photographs and video recording) and initial quantitative and qualitative analyses on non-preserved samples were performed using light microscopy (Zeiss inverted Axiovert 135, magnification 100x, 400x, and 1000x). Differential illumination contrast was used to permit a more detailed structural analysis of cells. Preserved samples were analyzed in more detail in the laboratory to confirm the on-board estimates. Organisms > 2 µm were identified at the lowest possible taxonomic level and from here on referred to as “species” although sometimes only higher taxonomic levels were identified. The complete list of identified species (taxa) was included in all analyses performed. Flagellates and miscellaneous organisms (size range 3–10 µm) were classified as autotrophs when chloroplasts were observed. The main taxonomic sources were Medlin and Priddle (1990), Tomas (1997 and references therein), Witkowski et al. (2000), and current and earlier versions of AlgaeBase (https://www.algaebase.org/). Species names and authorities were updated in February 2021, using AlgaeBase and WoRMS (http://www.marinespecies.org/index.php).
Biodiversity was calculated and presented in three ways (complete list in Table S2). Firstly, species number (species per sample) was provided for each station and depth where micro- and nanoplankton samples were collected. Secondly, “effective species number” was calculated for each sample by taking the exponential of Shannon entropy, i.e., effective species number = exp (Shannon index), based on Jost (2006) and Jost et al. (2010). The effective species number is an advantageous metric in field samplings by enabling comparison between samplings in terms of species diversity as it weights the number of species to its relative abundance, and in comparison to Shannon entropy, it obeys the doubling principle (Jost et al. 2010; Leinster and Cobbold 2012; Olofsson et al. 2020). Thirdly, the difference between effective species numbers and observed species in a given sample provides the number of rare species, i.e., the number of species only present in low abundance.
Chlorophyll a concentration
For Chl a analysis, 250–3000 ml of seawater from surface and deep samples (for depths see Table 2) were filtered onto GF/F filters, immediately frozen in liquid nitrogen (–196 °C) and stored in –80 °C. Filtration took place in dim light at + 4 °C to minimize the influence of light and temperature. For extraction, 1.5 ml of 100% MeOH was added, and the extraction and HPLC-analysis continued according to Wright and Jeffrey (1997) using an absorbance diode-array detector (Spectraphysics UV6000LP). The HPLC system was calibrated with pigment standards from DHI Lab, Denmark. Chl a is expressed as µg l−1.
Statistical analyses and data handling
Biodiversity calculations and non-metric multi-dimensional scaling (NMDS) plots were conducted using the package “vegan” in R (Oksanen et al. 2018; R Core Team 2018). All data were processed and plotted using the package “Tidyverse” in R (Wickham 2017). Pearson correlations were performed between species diversity metrics (species number in a sample, effective species number, rare species, and percent rare species) and latitude and cell abundance for the surface samples using Microsoft Excel 2020. NMDS plots were produced using the “metaMDS” function in R, which is optimized for community ecology data. We applied the function for species groups across surface stations and the “envfit” function to correlate it to environmental factors. The same procedure was also applied for diversity metrics across the surface stations and environmental factors. Significant correlations were set to p < 0.05.
Results
Station characteristics
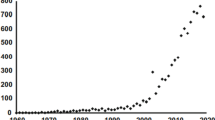
One hundred ninety-six different species/taxa (where of 73 were identified to species level) were observed during the expedition and demonstrate the diverse Arctic micro- and nanoplankton communities (Table S2). The number of species observed increased with the number of collected samples when traveling from north to south, with a polygonal curve fit (R2 = 0.994, n = 56; Fig. S1). However, there were some discontinuities of the curve related to distance but also reflecting the patchiness of the micro- and nanoplankton communities. This means that the total number of species continuously increased as entering new plankton communities, but at a decreasing rate. Communities consisted of cryptophytes, chrysophytes, diatoms, dinoflagellates, haptophytes, prasinophytes, raphidophytes, ciliates, (additional) autotrophic flagellates, heterotrophic flagellates, euglenophytes, choanoflagellates, and miscellaneous plankton of size range 3–10 µm (dominated by very small flagellates) (Fig. 2; Tables 3 and 4). The relative abundance of groups from the different samples indicated a large variation in community composition between stations (Fig. 3). The NMDS analysis on phytoplankton groups and environmental factors in surface samples demonstrated no significant associations (Fig. 4a).
The abundance of micro- and nanoplankton groups (cells l−1) at the different stations from the surface and deep sampling. Vertical dashed lines indicate transects 2a, 2b, 3, 4, 5, 6, 7, 8, and 9 (see Table 1 for details)
Inorganic nutrient concentrations were > 0.3 μM at all stations except two stations with high Chl a concentrations (> 9 μg l−1; Table 2). Similar nutrient concentrations at surface, and deep samples suggested a well-mixed upper water mass, a pattern mostly reflected also in the species distribution and Chl a concentrations (Fig. 2 and Table S3).
Micro- and nanoplankton diversity
The total number of observed species in each sample varied between 12 and 68, and the biodiversity expressed as effective species numbers ranged between 1.0 and 8.8 (Fig. 5 and Table 2), with no correlation between the two variables (Pearson, r = − 0.12, n = 56). The difference between effective species numbers and observed species indicates that 88 ± 8% of the species belonged to the rare fraction, i.e., a large number of species were present in a very low abundance (Fig. 5). The number of observed species was positively correlated to the number of rare species (Pearson, r = 0.99, n = 56), and number of observed species was also positively correlated to the percent rare species in a sample (Pearson, r = 0.63, n = 56). The total abundance and number of species found in a sample was also positively correlated (Pearson, r = 0.63, n = 56), as well as abundance and number of rare species (Pearson, r = 0.66, n = 56). There was a weak negative correlation between effective species number and total abundance in a sample (Pearson, r = − 0.40, n = 56) and a negative correlation between effective species number and percent rare species (Pearson, r = − 0.72, n = 56). The number of rare species was correlated with percent rare in sample (Pearson, r = 0.71, n = 56) but only weakly negative with effective species number (Pearson, r = − 0.24, n = 56).
Total number of species observed per sample as the sum of effective species numbers and rare species for each station from the surface and deeper samplings. Vertical dashed lines indicate transects 2a, 2b, 3, 4, 5, 6, 7, 8, and 9 (see Table 1 for details)
The percent rare species in a sample was associated to a combination of latitude, ice coverage, phosphate, and silicate, while abundance was partly associated with Chl a, and species number and rare species were associated with each other but with no environmental factors, and effective species numbers were not related to any environmental variables (NMDS analysis; Fig. 4b). The latitude vector pointed to the opposite direction as Chl a in the NMDS plot, and latitude was negatively correlated both to species number (Pearson, r = − 0.71, n = 56) and to rare species (Pearson, r = − 0.72, n = 56), while only weakly to percent (r = − 0.54, n = 56) and abundance (r = − 0.35, n = 56), suggesting higher Chl a and species numbers were observed further south. Ice presence was negatively correlated with both species number (r = − 0.57, n = 56) and rare species (r = − 0.57, n = 56), suggesting less species under the ice as compared with open water.
Micro- and nanoplankton abundance
At 11 stations, cell numbers were > 1 × 106 cells l−1 (Fig. 2, and Table S2) but were dominated by different species. For example, the most abundant species on station 33 was the haptophyte Phaeocystis sp., with up to 2.9 × 106 cells l−1 at the surface and 5.1 × 106 cells l−1 in the deep sampling, both as single cells and in colonies. Further, the most abundant species on stations 44 (both depths), 48 (surface), and 50 (both depths) were the small (1–3 µm) prasinophyte Micromonas sp., with cell numbers up to 4.0 × 106 cells l−1. Station 57 and 62 were dominated by diatoms, where Chaetoceros socialis was the most abundant diatom at station 57 with 4.0 × 106 cells l−1 (surface) and 8.0 × 106 cells l−1 (deep). Again, the most abundant species on stations 80, 86, and 95 at both sampling depths were Phaeocystis sp., with 29.0 × 106 cells l−1 at station 95 (surface). Station 95 had the lowest nitrate concentration (< 0.1 μM; Table 1) but was replete in silicate (not used by, e.g., Phaeocystis sp.).
Diatom distribution
Diatoms were found at all sampled stations (Tables 3 and 4). Particularly two centric genera were frequently observed in large numbers: Chaetoceros spp. and Thalassiosira spp. (Table S2), and pennate diatom genera such as Pseudo-nitzschia sp. and Fragilariopsis spp. were frequently observed with the highest abundance at station 95 surface (3.9 × 105 cells l−1) and station 57 deep (1.9 × 105 cells l−1), respectively. The nearby station 58 (not sampled for plankton) had the lowest silicate concentration (0.14 μM, Table 1) and Chl a concentration of 10.4 μg l−1 (Table 2). Resting spores of Chaetoceros socialis, Fragilariopsis oceanica, and several species of Thalassiosira were observed mainly at stations 80–95, with up to 8 × 104 spores l−1. Resting spores of F. oceanica and Thalassiosira spp. were also found at stations 33, 57, and 62, with no overall pattern in relation to ice coverage or temperatures.
Dinoflagellate distribution
Generally, dinoflagellates were observed in much lower abundances as compared with diatoms, prasinophytes, and haptophytes (Tables 3 and 4). In the surface samples, the maximum number of cells were found for species belonging to Gyrodinium and Gymnodinium, with ~ 2.0 × 104 cells l−1 at stations 68 and 91 (Table S2). Heterocapsa sp. and Peridiniella danica were occasionally observed in relatively large numbers as compared with other dinoflagellates, while Katodinium glaucum was the most recurrent identifiable species, present at 18 stations (< 100 cells l−1). Naked (unidentified) dinoflagellates in the size range 8–60 µm were spread over all stations up to ~ 1.6 × 104 cells l−1. Thecate (unidentified) dinoflagellates occurred in different sizes and abundances, with up to 2.4 × 104 cells l−1 but slightly more frequently with lower latitudes and higher light intensities (towards the end of the expedition).
Species observed only south of 70° N
To reveal species with potential to establish further north if conditions change in their favor, we summarized all species only observed at stations below 70° N and at a minimum of three times. These species included the diatoms Neodenticula seminae in 6 samples, and Ephemera planamembranacea, Leptocylindrus minimus, Rhizosolenia cf. hebetata, Thalassionema sp., Corethron sp., Cylindrotheca sp., resting spores of Chaetoceros socialis, resting spores of Thalassiosira cf. nordenskioeldii, the dinoflagellate Protoperidinium depressum, the ciliate cf. Leegaardiella sol, colonies of choanoflagellates, and Meringosphera sp. Rare encounters in the samples included the dinoflagellate Pronoctiluca spinifera, which to our knowledge has not been observed in the Arctic region before this expedition.
Discussion
The Arctic region is negatively affected by climate change faster than any other ecosystem on Earth, and due to its early onset of climate effects, it may act as a model system for future changes of other ecosystems. Here, we demonstrated that micro- and nanoplankton community composition during early bloom conditions along the ice edge of Greenland consists of a highly diverse pool of species. The field campaign was conducted nearly two decades ago, providing an opportunity to use this dataset to reveal newly introduced species via shipping, currents, range expansions, or migration from southern warmer waters, as sea ice retreats and sea surface temperatures increase.
We observed more than 196 different species (taxa) of micro- and nanoplankton, which is within the range of what has been observed during Arctic summer (145 species, coastal Greenland, Krawczyk et al. 2015a; 153 species, Western Arctic, Wang et al. 2018), winter (145 species, Beaufort Sea, Niemi et al. 2011), and late spring (212 species, Yermak Plateau, Assmy et al. 2017). Herein, species number, observed species in each sample, varied between 12 and 68, which is in the upper range of the 7–12 observed by Niemi et al. (2011) and 10–30 by Assmy et al. (2017). The lower number of observed species in the samples by the mentioned studies can partly be related to different sampling volumes (50 and 250 ml, versus 1 L, respectively), but also differences between “under ice” and open water samplings. Even if it is challenging to compare studies, due to methodological variation, knowledge on species presence at a given time is still important as it may indicate an early introduction. Furthermore, comparisons among datasets are challenging due to continuous changes in nomenclature and the merging or splitting of species. One example is Skeletonema costatum where Sarno et al. (2005) introduced four new species with very similar morphology within the genus Skeletonema. In our dataset from 2002, we use S. costatum, a species recognized as cosmopolitan but rarely observed in the Arctic (Copepedia 2021). However, detecting change in phytoplankton communities relies on both species numbers and community structures (Dornelas et al. 2014; Hillebrand et al. 2018). Effective species numbers ranged from 1.0 to 8.8 (mean of 3.4), with no specific temporal or spatial variation and no correlation with number of observed species in a sample, rare species, or total abundance. Effective species numbers were in the same range as observed by Assmy et al. (2017) with a mean of ~ 5.4 (re-calculated by the present authors). Providing the total number of species alongside effective species number can be useful when describing the structure of an ecosystem since it will reveal the number of rare species.
Overall, about 88% of the species were considered rare species, and the number of rare species was positively correlated with both species number and total abundance, suggesting that a higher cell abundance in general involve higher number of species and more rare species. In addition, rare species and species numbers were negatively correlated with latitude, suggesting richer communities further south. Rare species have the potential to increase in abundance and their inclusion in monitoring studies can provide early indications of invasive species not yet established. They are also important under variable environmental conditions, where a decreased diversity can result in a less resilient ecosystem due to fewer species in each functional group under species loss (Snoeijs-Lejonmalm 2017). The Arctic ecosystem is undergoing major changes, not yet visible without a more detailed examination, and it is therefore of uttermost importance in future studies to also include quantification of rare species to determine if present day micro- and nanoplankton community already has less rare species as compared with two decades ago.
Until recently under-ice primary productivity has been underestimated due to the assumption of too low light intensities. However, massive blooms found under the sea ice indicated high productivity early in the spring, sometimes larger than in the open water (Arrigo et al. 2012; 2014; Arrigo and van Dijken 2015; Assmy et al. 2017), while Ardyna et al. (2011) observed higher Chl a concentrations with decreasing ice cover. Our measurements were performed during an early bloom, as indicated by generally high nutrient availability and only occasionally high biomass, in both ice covered and open waters (Chl a, Table 2). For example, stations like 80–82, 86, and 95 were sampled later in May and thereby had more developed blooms. This is similar to blooms observed north of Svalbard from the 25th of May and onwards, followed by a decrease in surface nitrate concentrations (Assmy et al. 2017). The number of species observed in each sample was correlated with the presence of ice, with a lower species number under the ice as compared with in the open water. One reason could be that under-ice algae often are dominated by specialists, e.g., species with a narrow range of environmental conditions they thrive in. The Arctic spring bloom, including ice-associated microalgae is subject for a mismatch in timing of carbon transfer to higher trophic levels (e.g., Søreide et al. 2010) and, thus, important to study for projecting future situations in timing, magnitude, and community composition.
Monitoring the establishment of potentially harmful non-native species and early shifts in community composition is important from an ecosystem management perspective, although it is not necessarily disadvantageous if newly introduced species serves a redundant function in the food web or occupy the same niche. As the sea ice declines, microplankton can travel with ballast water through the Arctic, with future projected routes from North America passing the west coast of Greenland and to Russia or Japan (Melia et al. 2016). Increased influence of Pacific water via the Arctic Ocean due to less sea ice and/or changed ocean circulation in the Arctic Ocean was exemplified by the diatom, Neodenticula seminae (Miettinen et al. 2013). During 1999 this species was observed in the Laborador Sea for the first time in 800,000 years (Reid et al. 2007). The reintroduction from the Pacific Ocean might be coupled to an extraordinary warm year in 1998 with more near-surface water transport from the Pacific to the Atlantic (Arrigo and van Dijken 2004; Jones et al. 2003; Poulin et al. 2010). We observed N. seminae in six samples collected between 65 and 68° N and sediment samples collected during 2006–2008 indicated an establishment of N. seminae all the way up to 79° N (Miettinen et al. 2013), which indicates an ongoing northward spread of the species since 1999. Since N. seminae may account for > 40% of the diatom assemblages in the sub-arctic Pacific (Shimada et al. 2006) and usually dominate blooms in the Gulf of St. Lawrence (Sakshaug et al. 2009), this might reflect the future situation further north. The potential effect on the northern ecosystem is, however, still unknown but should be under observation.
Further, the diatom Leptocylindrus minimus was observed in samples collected at stations south of 70° N. This diatom has been associated with fish mortalities (Clement and Lambeye 1991) and is common in the Canadian Bay of Fundy (Martin et al. 2010), coastal waters in general (Horner 2002), and in Northern European seas (Kraberg et al. 2010). L. minimus was also observed at 64° N, SW Greenland, in samples from 2006 to 2010 (Krawczyk et al. 2015b). It has never, to our knowledge, been observed in the Arctic region (north of 70°). L. minimus is generally favored by high nitrate concentrations (Horner 2002; Alves-de-Souza et al. 2008), high temperature, and high salinity (Pizarra et al. 1997). Since nitrate was available at all stations herein except 95 (Table 2), future physical changes, e.g., elevated temperatures in this region, may govern its northward spread during spring-bloom conditions. Another example of a species observed for the first time in the Artic region to our knowledge was the heterotrophic dinoflagellate Pronoctiluca spinifera that generally thrives in eutrophic conditions replete with prey (Gomez 2013). Establishment of other introduced species can be expected with the on-going changes, potentially affecting the existing phytoplankton communities by altering local food webs and species dynamics, with possible implications for carbon cycling through feeding patterns or altered primary productivity. Additional species only observed below 70° N (this study) included Rhizosolenia cf. hebetata; however, different varieties of R. hebetata were observed in sediment traps around 72°N already 1991–1992 (Kohly 1998). Later, R. hebetata (and varieties) was observed in West Spitsbergen waters around 79° N (2007 and 2010, Kubiszyn et al. 2014) and SW Greenland (2013, Krawczyk et al. 2018). Another example is Ephemera planamembranacea, a diatom observed in the Gulf of St. Lawrence, around 50° N (Bérard-Therriault et al. 1999), but also recorded from the Antarctic (Scott and Thomas 2005); thus, there is no a priori reason to believe it should not thrive in the Arctic. These are few examples to highlight the importance of more frequent sampling and/or to scrutinize existing datasets in order to monitor rapid changes in the basis of the Arctic food web.
Marine phytoplankton primary production is globally considered nitrogen limited (Moore et al. 2013). Concentrations of nitrate were herein replete for the phytoplankton during this early stage of the spring bloom except in station 95. This pattern is confirmed by Assmy et al. (2017) demonstrating a decrease in nitrate concentration later in the season for the region north of Svalbard. Silicate concentrations were > 2 μM except in station 58, having high diatom abundance and only 0.14-μM silicate, suggesting it was high enough for diatom frustule formation. In addition, phosphate concentrations were > 0.25 μM at all locations, and with the lowest concentration at the station with the highest Chl a concentration. The NMDS analyses suggested that a combination of high phosphate and silicate concentrations as well as high latitude and ice presence were associated with percent rare species and community composition in some of the stations (Fig. 4b). However, since nutrients were replete at almost all stations and both ice presence and latitude were rather negatively correlated with percent rare species, this combination of parameters does not provide any further insight as potential drivers of the community composition.
Further understanding of potential climate change effects on micro- and nanoplankton in the future Arctic seas is fundamental. As demonstrated herein, we already observed changes in the microplankton community due to warming of the region, with, e.g., species spreading from the North Pacific to the North Atlantic. We define a baseline of micro- and nanoplankton diversity two decades ago from which we may reveal changes and potential biodiversity losses in these communities. Since low diversity might affect the resilience of an ecosystem, a decrease in rare species could possibly make the ecosystem more vulnerable to changes. Indeed monitoring is needed for early warning of potential threats and to guide research and policy and management responses accordingly. By combining microscopy with molecular techniques, it may help to determine rare species as well as small size species (e.g., picoplankton), which may be of importance in terms of early introduction of non-native species. Although identifying and monitoring species abundance may come with a high cost, the costs of controlling already established and harmful organisms might be even higher.
References
Alves-de-Souza C, González MT, Iriarte JL (2008) Functional groups in marine phytoplankton assemblages dominated by diatoms in fjords of southern Chile. J Plankton Res 30:1233–1243
Ardyna M, Gosselin M, Michel C, Poulin M, Tremblay J-E (2011) Environmental forcing of phytoplankton community structure and function in the Canadian High Arctic: contrasting oligotrophic and eutrophic regions. Mar Ecol Prog Ser 442:37–57
Arrigo KR, van Dijken GL (2004) Annual cycles of sea ice and phytoplankton in Cape Bathurst polynya, southeastern Beaufort Sea. Canadian Arctic Geophys Res Lett 31:L08304
Arrigo KR et al (2012) Massive phytoplankton blooms under Arctic sea ice. Science 336:1408–1408
Arrigo KR et al (2014) Phytoplankton blooms beneath the sea ice in the Chukchi Sea. Deep-Sea Res PT II 105:1–16
Arrigo KR, van Dijken GL (2015) Continued increases in Arctic Ocean primary production. Prog Oceanogr 136:60–70
Assmy P et al (2017) Leads in Arctic pack ice enable early phytoplankton blooms below snow-covered sea ice. Sci Rep 7:40850
Bauerfeind E, Bodungen B, Arndt K, Koeve W (1997) Particle flux, and composition of sedimenting matter, in the Greenland Sea. J Mar Syst 5:411–423
Bérard-Therriault L, Poulin M, Bossé L (1999) Guide d’identification du phytoplancton marin de l’estuaire et du golfe du Saint-Laurent incluant également certains protozoaires. Publi Spéc Can Sci Halieut Aquatic 128:387
Booth BC, Smith WO Jr (1997) Autotrophic flagellates and diatoms in the Northeast Water Polynya, Greenland: summer 1993. J Mar Syst 10:241–261
Chan FT et al (2019) Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob Change Biol 25:25–38
Clement A, Lembeye G (1991) Phytoplankton monitoring program in the fish farming region of South Chile. University of Rhode Island/et al. 5th International Conference on Toxic Marine Phytoplankton, Newport (Elsevier). pp 223–223.
Cleve PT (1873) On diatoms from the Arctic Sea. Beh Kgl Svenska VetenskAkad Handl 1:1–28
Copepedia https://www.st.nmfs.noaa.gov/nauplius/media/copepedia/taxa/T2000035/html/nicheframe.html retrieved April 2021
Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science 344:296–299
Gómez F (2013) Morphology and distribution of Pronoctiluca (Dinoflagellata, incertae sedis) in the Pacific Ocean. Acta Oceanol Sin 32:71–76
Gordon LI, Jennings JC Jr, Ross AA, Krest JM (1993) A suggested protocol for continuous flow automated analysis of seawater nutrients (Phosphae, Nitrate, Nitrite and Silicic Acid) in the WOCE hydrographic program and the joint global ocean fluxes study. WOCE Hydrographic Program Office Methods Manual WHPO 68/91, (Paris: WOCE Hydrographic Programme Office), pp 1–52
Gradinger RR, Baumann MEM (1991) Distribution of phytoplankton communities in relation to the large-scale hydrographical regime in the Fram Strait. Mar Biol 111:311–321
Hillebrand H et al (2018) Biodiversity change is uncoupled form species richness trends: Consequences for conservation and monitoring. J Appl Ecol 55:169–184
Hooper DU et al (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Horner RA (2002) A taxonomic guide to some common phytoplankton. Biopress Limited, Dorset Press, Dorchester, UK, p 195
IPCC (2018) Summary for Policymakers. In: Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [Masson-Delmotte V. et al. (eds.)]. World Meteorological Organization, Geneva, Switzerland, 32 pp.
Jones EP, Swift JH, Anderson LG, Lipizer M, Civitarese G, Falkner KK, Kattner G, McLaughlin F (2003) Tracing Pacific water in the North Atlantic Ocean. J Geophys Res 108(C4):3116
Jost L (2006) Entropy and diversity. Oikos 113(2):363–375
Jost L, DeVries P, Walla T, Greeney H, Chao A, Ricotta C (2010) Partitioning diversity for conservation analyses. Div Distrib 16:65–76
Kannan R, James DA (2009) Effects of climate change on global biodiversity: a review of key literature. Trop Ecol 50(1):31–39
Karlusich JJP, Ibarbalz FM, Bowler C (2020) Phytoplankton in the Tara Ocean. Ann Rev Mar Sci 12:233–265
Kilias E, Kattner G, Wolf C, Frickenhaus S, Metfies K (2014) A molecular survey of protist diversity through the central Arctic Ocean. Pol Biol 37:1271–1287
Kohly A (1998) Diatom flux and species composition in the Greenland Sea and the Norwegian Sea in 1991–1992. Mar Geol 145:293–312
Kraberg A, Baumann M, Durselen CD (2010) Coastal phytoplankton: Photo guide for Northern European Seas. Verlag Dr, Friedrich Pfeil, Munchen, Germany, p 204
Krawczyk DW, Arendt KE, Juul-Pedersen T, Sejr MK, Blicher ME, Jakobsen HH (2015a) Spatial and temporal distribution of planktonic protists in the East Greenland fjord and offshore waters. Mar Ecol Prog Ser 538:99–116
Krawczyk DW, Witkowski A, Juul-Pedersen T, Arendt KE, Mortensen J, Rysgaard S (2015b) Microplankton succession in a SW Greenland tidewater glacial ford infuenced by coastal infows and runof from the Greenland Ice Sheet. Polar Biol 38:1515–1533
Krawczyk DW, Meire L, Lopes C et al (2018) Seasonal succession, distribution, and diversity of planktonic protists in relation to hydrography of the Godthåbsfjord system (SW Greenland). Polar Biol 41:2033–2052
Kubiszyn AM, Piwosz K, Wiktor JM Jr, Wiktor JM (2014) The effect of inter-annual Atlantic water inflow variability on the planktonic protist community structure in the West Spitsbergen waters during the summer. J Plankton Res 36:1190–1203
Lafond A et al (2019) Late spring bloom development of pelagic diatoms in Baffin Bay. Elem Sci Anth 7:44
Lebrun M, Vancoppenolle M, Madec G, Massonnet F (2019) Arctic sea-ice-free season projected to extend into autumn. Cryosphere 13:79–96
Leinster T, Cobbold CA (2012) Measuring diversity: the importance of species similarity. Ecology 93(3):477–489
Martin J, Tremblay JÉ, Gagnon J, Tremblay G, Lapoussière A, Jose C, Poulin M, Gosselin M, Gratton Y, Michel C (2010) Prevalence, structure and properties of subsurface chlorophyll maxima in Canadian Arctic waters. Mar Ecol Prog Ser 412:69–84
Medlin LK, Priddle J (1990) Polar marine diatoms. British Antarctic Survey, Cambridge, p 214
Melia N, Haines K, Hawkins E (2016) Sea ice decline and 21st century trans-Arctic shipping routes. Geophys Res Lett 43:9720–9728
Miettinen A, Koç N, Husum K (2013) Appearance of the Pacific diatom Neodenticula seminae in the northern Nordic Seas – An indication of changes in Arctic sea ice and ocean circulation. Mar Micropaleontol 99:2–7
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6:485–492
Moore CM et al (2013) Processes and patterns of oceanic nutrient limitation. Nat Geosci 6:701–710
Nehring S (1998) Establishment of thermophilic phytoplankton in the North Sea: biological indications of climate change? ICES J Mar Sci 55:818–823
Niemi A, Michel C, Hille K, Poulin M (2011) Protist assemblages in winter sea ice: setting the stage for the spring ice algal bloom. Pol Biol 34:1803–1817
Nilsson J, Björk G, Rudels B, Winsor P, Torres D (2008) Liquid freshwater transport and Polar Surface Water characteristics in the East Greenland Current during the AO-02 Oden expedition. Progr Oceanogr 78:45–57
Oksanen J, et al. (2018) vegan: Community Ecology Package. R package version 2.5–3. https://CRAN.R-project.org/package=vegan. Retrieved April 2021
Olofsson M, Hagan J, Karlson B, Gamfeldt L (2020) Large seasonal and spatial variation in nano- and microphytoplankton diversity along a Baltic Sea – North Sea salinity gradient. Sci Rep 10: 17666
Owrid G, Socal G, Civitarese G, Luchetta A, Wiktor J, Nöthig E-M, Andreassen I, Schauer U, Strass V (2000) Spatial variability of phytoplankton, nutrients and new production estimates in the waters around Svalbard. Polar Res 19:155–171
Pillai P, Gouhier TC (2019) Not even wrong: The spurious measurement of biodiversity´s effect on ecosystem functioning. Ecology 100(7):e02645
Pizarra G, Guzman L, Frangopulas M, Alarcon C (1997) Environmental conditions associated with phytoplankton blooms in a remote area of PSP detection (Bahia Pecket, strait of Magellan, Chile). VIII International conference on Harmful algae - Abstracts and Posters Classification. Instituto Español de Oceanografia, Centro Oceanografico de Vigo, Vigo
Poulin M, Lundholm N, Bérard-Therriault L, Starr M, Gagnon R (2010) Morphological and phylogenetic comparisons of Neodenticula semina (Bacillariophyta) populations between the subarctic Pacific and the Gulf of St Lawrence. Eur J Phycol 45(2):127–142
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R version 3.5.1, https://www.R-project.org/. Retrieved April 2021
Ratkova TN, Wassmann P (2002) Seasonal variation and spatial distribution of phyto- and protozooplankton in the central Barents Sea. J Mar Syst 38:47–75
Reid PC, Johns DG, Edwards M, Starr M, Poulin M, Snoeijs P (2007) A biological consequence of reducing Arctic ice cover: arrival of the Pacific diatom Neodenticula seminae in the North Atlantic for the first time in 800 000 years. Glob Change Biol 13:1910–1921
Renaut S, Devred E, Babin M (2018) Northward expansion and intensification of phytoplankton growth during the early ice-free season in Arctic. Geophys Res Lett 45:10590–10598
Ricciardi A et al (2017) Invasion science: A horizonscan of emerging challenges and opportunities. Trends Ecol Evol 32:464–474
Rudels B, Björk G, Nilsson J, Winsor P, Lake I, Nohr C (2005) The interaction between waters from the Arctic Ocean and the Nordic Seas north of Fram Strait and along the East Greenland Current: results from the Arctic Ocean-02 Oden expedition. J Mar Syst 55:1–30
Sakshaug E, Johnsen G, Kristiansen S, von Quillfeldt C, Rey F, Slagstad D, Thingstad F (2009) Phytoplankton and primary production. In: Sakshaug E, Johansen G, Kovacs K (eds) Ecosystem Barents Sea. Tapir Academic Press, Trondheim, pp 167–208
Samtleben C, Scäfer P, Andruleit H, Baumann A, Baumann K-H, Kohly A, Matthiessen J, Schröder-Ritzrau A (1995) Plankton in the Norwegian-Greeland Sea: from living communities to sediment assemblages – an actualistic approach. Geol Rundsch 84:108–136
Sarno D, Kooistra WHCF, Medlin LK, Percopo I, Zingone A (2005) Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species1. J Phycol 41:151–176
Scott FJ, Thomas DP (2005) Diatoms. In: Scott FJ, Marchant HJ (eds) Antarctic Marine Protists Australian Biological Resources Study. Canberra and Australian Antarctic Division, Hobart, pp 13–201
Sergeeva VM, Zhitina LS, Mosharov SA, Nedospasov AA, Polukhin AA (2018) Phytoplankton community structure in the polar front of the Eastern Barents Sea at the end of the growth season. Mar Biol 58(5):700–709
Shimada C, Tanaka Y, Tanimura Y (2006) Seasonal variation in skeletal silicification of Neodenticula seminae, a marine planktonic diatom: sediment trap experiments in the NW Pacific Ocean (1997–2001). Mar Micropaleontol 60:130–144
Sjöstedt J, Koch-Schmidt P, Pontarp M, Canbäck B, Tunlid A, Lundberg P, Hagström Å, Riemann L (2012) Recruitment of members from the rare biosphere of marine bacterioplankton communities after an environmental disturbance. Appl Environ Microbiol 78:1361–1369
Snoeijs-Leijonmalm P (2017) Patterns of biodiversity. In: Snoeijs-Leijonmalm P, Schubert H, Radziejewska T (eds) Biological Oceanography of the Baltic Sea. Springer Science Business Media, Dordrecht, pp 123–191
Steffen W et al (2015) Planetary boundaries: Guiding human development on a changing planet. Science 347:1259855
Søreide J, Leu E, Berge J, Graeve M, Falk-Petersen S (2010) Timing in blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob Change Biol 16:3154–3163
Tomas CR (ed) (1997) Identifying marine phytoplankton. Academic Press, San Diego, 858
Tuschling K, Juterzenka VK, Okolodkov YB, Anoshkin A (2000) Composition and distribution of the pelagic and sympagic algal assemblages in the Laptev Sea during autumnal freeze-up. J Plankton Res 22(5):843–864
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int Verein Theor Angew Limnol 29:117–126
Von Quillfeldt C (1997) Distribution of diatoms in the Northeast Water Polynya, Greenland. J Mar Syst 10:211–240
Wang Y, Xiang P, Kang J-h, Ye Y-y, Lin G-m, Yang Q-l, Lin M (2018) Microphytoplankton community structure in the western Arctic Ocean: surface layer variability of geographic and temporal considerations in summer. Hydrobiologica 811:295–312
Wassmann P, Reigstad M (2011) Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. Oceanography 24(3):220–231
Wickham H (2017) Tidyverse: Easily Install and Load the 'Tidyverse'. R package version 1.2.1. https://CRAN.R-project.org/package=tidyverse. Retrieved April 2021
Witkowski A, Lange-Bertalot H, Metzeltin D (2000) Diatom flora of marine coasts I. Iconogr Diatomol 7:1–925
Wright SW, Jeffrey SW (1997) High-resolution HPLC system for chlorophylls and carotenoids of marine phytoplankton. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris, pp 327–342
Acknowledgements
The authors would specifically acknowledge our colleague, late Dr. Mats Kuylenstierna, who performed the phytoplankton analyses. He is greatly missed. We wish to thank the Swedish Polar Secretariat for their logistic support, the Captain and crew of I/B Oden for their assistance during the cruise, everybody running the rosette sampler and performed on-board analyses of e.g., salinity and temperature. Special thanks to A. Engelsen for assisting wherever needed, to S. Becker, UCSD/SIO Oceanographic Data Facility for the nutrient data, and to Jeremy Schreier for proof-reading.
Funding
Open access funding provided by University of Gothenburg. This research has been supported by grants to AW from the Swedish Polar Research Secretariat, the YMER-80 Foundation, the Lennander Foundation, the Lars Hierta Memorial Foundation, and Wilhelm and Martina Lundgren’s Science Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal testing was performed during this study.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The cruise and sampling permits were administered by the Swedish Polar Secretariat.
Data availability
The data is available from the authors upon request.
Authors’ contributions
AW performed sampling and collected the data during the expedition and was responsible for analyses and planning of the phytoplankton part of the field survey. MO processed and plotted the data and wrote a draft version of the manuscript. Both authors contributed during the writing process and have approved the final version of the manuscript.
Additional information
Communicated by B. Beszteri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olofsson, M., Wulff, A. Looking back to the future—micro- and nanoplankton diversity in the Greenland Sea. Mar. Biodivers. 51, 61 (2021). https://doi.org/10.1007/s12526-021-01204-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01204-w