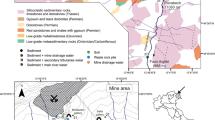
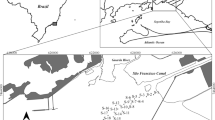
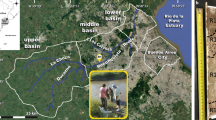
Abstract
The concentration of 14 potentially toxic elements (PTE), including emerging contaminants, was determined in mine tunnel sludges, streambed sediments, and in the bank-full channel load of the Baccatoio stream, in Tuscany (Italy), in a catchment affected by AMD. The geochemical profile of streambed piston-cored sediments and the solute concentrations of the hyporheic pore water and surface water were also determined. Sediments are characterized by high concentrations of As, Cd, Tl, Sb, Ni, Cu, Zn, besides Al, Mn, and Fe. The As and Fe are closely coupled, indicating that iron oxyhydroxide precipitation plays a major role in controlling As sequestration. Mn oxides likely act as a sorbent for Zn, Ni, Sb, and Pb. Downstream geochemical trends indicate that precipitation and sorption represent the natural attenuation processes controlling the PTE loading released from the contaminated acidic effluents. The obtained results highlight that the Baccatoio stream sediments may act as secondary pollution sources via redox cycling, allowing contaminant remobilization to surface- and ground-water across the streambed, affecting the stream environment and aquatic biota.
Zusammenfassung
Die Konzentrationen von 14 potentiell toxischen Elementen (PTE) einschließlich neu auftretender Schadstoffe wurde in Schlamm aus Bergbaustollen, Sedimenten aus einem Bachbett und aus dem Hauptkanal des Baccatoio Bachs in einem durch AMD geprägten Einzugsgebiet in der Toskana (Italien), untersucht. Darüber hinaus wurde auch das geochemische Profil von mittels Kolbenkernprobenehmer entnommener Sedimente sowie die gelösten Konzentrationen des hyporheisches Porenwassers und Oberflächenwassers bestimmt. Die Sedimente werden neben Al, Fe und Mn durch hohe Konzentrationen von As, Cd, Cu, Ni, Sb, Tl und Zn charakterisiert. Die enge Verknüpfung von As und Fe weist darauf hin, dass die Ausfällung von Eisenoxyhydroxiden eine entscheidende Rolle bei der Abscheidung von As spielt. Manganoxide sind mutmaßlich ein Sorbent für Ni, Pb, Sb und Zn. Geochemische Trends entlang des Fließpfades weisen darauf hin, dass die natürliche Ausfällung und Sorption die stromab gerichtet Befrachtung des Fließgewässers mit PTE aus den kontaminierten, sauren Abflüssen bestimmen. Die Ergebnisse der Studie zeigen, dass die Baccatoio Sedimente infolge natürlicher Redoxprozesse als sekundäre Schadstoffquellen wirken können. Dies ermöglicht eine Schadstoff-Remobilisierung in die Grund- und Oberflächenwasserressourcen im Bereich des Bachbetts, welche die Fließgewässerökologie und aquatischen Biota beeinträchtigt.
Resumen
Se determinó la concentración de 14 elementos potencialmente tóxicos (ETP), incluidos los contaminantes emergentes, en los lodos de los túneles de la mina, en los sedimentos del cauce y en la carga del cauce del arroyo Baccatoio, en la Toscana (Italia), en una cuenca afectada por AMD. También se determinó el perfil geoquímico de los sedimentos con núcleo de pistón del arroyo y las concentraciones de solutos del agua de poro hiporreica y del agua superficial. Los sedimentos se caracterizan por sus altas concentraciones de As, Cd, Tl, Sb, Ni, Cu, Zn, además de Al, Mn y Fe. El As y el Fe están estrechamente acoplados, lo que indica que la precipitación de oxihidróxido de hierro desempeña un papel importante en el control del secuestro de As. Los óxidos de Mn probablemente actúan como sorbentes para el Zn, Ni, Sb y Pb. Las tendencias geoquímicas aguas abajo indican que la precipitación y la sorción representan los procesos naturales de atenuación que controlan la carga de TEP liberada por los efluentes ácidos contaminados. Los resultados obtenidos destacan que los sedimentos del arroyo Baccatoio pueden actuar como fuentes secundarias de contaminación a través del ciclo redox, permitiendo la removilización de contaminantes a las aguas superficiales y subterráneas a través del lecho del arroyo, afectando al medio ambiente del arroyo y a la biota acuática.
抽象的
测定了意大利托斯卡纳(Tuscany)地区受酸性矿山废水(AMD)影响的Baccatoio河的河道淤泥, 河床沉积和平滩河道泥沙的14种潜在有毒元素(PTE)和新出现污染物的浓度. 测定了河床沉积物柱状岩芯的地球化学剖面以及潜流孔隙水和地表水的溶质浓度. 河床沉积物特征表现为除Al、Mn和Fe外, 还含有高浓度As, Cd, Tl, Sb, Ni, Cu和Zn. As与Fe密切耦合, 表明铁氢氧化物的沉淀过程对As捕获封存起控制作用. 锰氧化物很可能是Zn, Ni, Sb和Pb的吸附剂. 下游地球化学趋势表明, 沉淀和吸附是控制酸性污染废水(AMD)释放潜在有毒元素(PTE)负荷的主要自然衰减过程. 研究进一步表明, Bacatoio河流沉积物可能通过氧化-还原循环作用成为二次污染源, 污染物穿过河床再次迁移到地表水和地下水, 影响河流环境和水生生物.




Similar content being viewed by others
References
Asta MP, Ayora C, Román-Ross G, Cama J, Acero P, Gault AG, Charnock JM, Bardelli F (2010) Natural attenuation of arsenic in the Tinto Santa Rosa acid stream (Iberian Pyritic Belt, SW Spain): the role of iron precipitates. Chem Geol 271:1–12
Barbieri M, Masi U, Tolomeo L (1982) Strontium geochemistry in the epithermal baryte deposits from the Apuan Alps (northern Tuscany, Italy). Chem Geol 35:351–356
Bigham JM, Nordstrom DK (2000) Iron and aluminum hydroxysulfates from acidic sulfate waters. Rev Mineral Geochem 40:351–403
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG, Paktunc D, Gould WD, Johnson DB (2014) The geochemistry of acid mine drainage. Treatise Geochem 11:131–190 (2nd edition)
Boulton AJ, Datry T, Kasahara T, Mutz M, Stanford JA (2010) Ecology and management of the hyporheic zone: stream-groundwater interactions of running waters and their floodplains. J N Am Benthol Soc 29:26–40
Campanella B, Onor M, D’Ulivo A, Giannecchini R, D’Orazio M, Petrini R, Bramanti E (2016) Human exposure to thallium through tap water: a study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy). Sci Tot Env 548–549:33–42
Campanella B, Colombaioni L, Benedetti E, Di Ciaula A, Ghezzi L, Onor M, D’Orazio M, Giannecchini R, Petrini R, Bramanti E (2019) Toxicity of thallium at low doses: a review. Int J Environ Res Public Health 16(23):4732. https://doi.org/10.3390/ijerph16234732
Cidu R, Dadea C, Desogus P, Fanfani L, Manca PP, Orrù PP (2012) Assessment of environmental hazards at abandoned mining sites: a case study in Sardinia, Italy. Appl Geochem 27:1795–1806
Cornel RM, Schwertmann U (1996) The iron oxides. VCH Weinheim, Germany, pp 349–373
Corson-Rikert HA, Wondzell SM, Haggerty R, Santelmann MV (2016) Carbon dynamics in the hyporheic zone of a headwater mountain stream in the Cascade Mountains, Oregon. Water Resour Res 52:7556–7576
Cranswick RH, Cook PG, Lamontagne S (2014) Hyporheic zone exchange fluxes and residence times inferred from riverbed temperature and radon data. J Hydrol 519:1870–1881
D’Orazio M, Biagioni C, Dini A, Vezzoni S (2017) Thallium-rich pyrite ores from Apuan Alps, Tuscany, Italy: constraints for their origin and environmental concerns. Miner Deposita 52:687–707
D’Orazio M, Campanella B, Bramanti E, Ghezzi L, Onor M, Vianello G, Vittori-Antisari L, Petrini R (2020) Thallium pollution in water, soils and plants from a past-mining site of Tuscany: sources, transfer processes and toxicity. J Geochem Explor 209:106434
De Giudici G, Pusceddu C, Medas D et al (2017) The role of natural biogeochemical barriers in limiting metal loading to a stream affected by mine drainage. Appl Geochem 76:124–135
De Giudici G, Medas D, Cidu R et al (2019) Assessment of origin and fate of contaminants along mining-affected Rio Montevecchio (SW Sardinia, Italy): a hydrologic-tracer and environmental mineralogy study. Appl Geochem 109:104420
Della Puppa L, Komárek M, Bordas F, Bollinger JC, Joussein E (2013) Adsorption of copper, cadmium, lead and zinc onto synthetic manganese oxide. J Colloid Interface Sci 399:99–106
Deng Y (1997) Formation of iron(III) hydroxides from homogeneous solutions. Water Res 31:1347–1354
Dore E, Fancello D, Rigonat N et al (2020) Natural attenuation can lead to environmental resilience in mine environment. Appl Geochem 117:104597
Doveri M, Menichini M, Cerrina Feroni A (2013) Stable water isotopes as fundamental tool in karst aquifer studies: some results from isotopic applications in the Apuan Alps carbonatic complexes (NW Tuscany). Italian J Eng Geol Environ 1:33–50
Doveri M, Stenni B, Petrini R, Giannecchini R, Dreossi G, Menichini M, Ghezzi L (2019) Oxygen and hydrogen isotopic composition of waters in a past-mining area of southern Apuan Alps (Italy): hydrogeological characterization and implications on the fate of potentially toxic elements. J Geochem Explor 205:106338
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley-Interscience, New York City
EPA (2006) SW-486: test methods for evaluating solid waste, physical/chemical methods. U.S. Environmental ProtectionAgency, http://www.epa.gov/epaoswer/hazwaste/test/sw486.htm
Farjana SH, Huda N, Parvez Mahmud MA, Saidur R (2019) A review on the impact of mining and mineral processing industries through life cycle assessment. J Clean Prod 231:1200–1217
Findlay S (1995) Importance of surface-subsurface exchange in stream ecosystems: the hyporheic zone. Limnol Oceanogr 40:159–164
Fisher MM, Brenner M, Reddy KE (1992) A simple, inexpensive piston corer for collecting undisturbed sediment/water interface profiles. J Paleolimnol 7:157–161
Ford RG, Bertsch PM, Farley KJ (1997) Changes in transition and heavy metal partitioning during hydrous oxide aging. Environ Sci Technol 31:2028–2033
Förstner U, Wittman G (1981) Metal pollution in the aquatic environment. Springer Verlag, New York City
Frau F, Cidu R, Casu M, Soriga A (2019) Assessing arsenic sources in landfill areas: a case study in Sardinia. Ital J Geosci 138:116–123
Fuller CC, Baragar JR (2014) Processes of zinc attenuation by biogenic manganese oxides forming in the hyporheic zone of Pinal Creek, Arizona. Environ Sci Technol 48:2165–2172
Galán E, Gómez-Ariza JL, González I, Fernández-Caliani JC, Morales E, Giráldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite belt. Appl Geochem 18:409–421
George LL, Biagioni C, D’Orazio M, Cook NJ (2018) Textural and trace element evolution of pyrite during greenschist facies metamorphic recrystallization in the southern Apuan Alps (Tuscany, Italy): influence on the formation of Tl-rich sulfosalt melt. Ore Geol Rev 102:59–105
Ghezzi L, D’Orazio M, Doveri M, Lelli M, Petrini R, Giannecchini R (2019) Groundwater and potentially toxic elements in a dismissed mining area: thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J Geochem Explor 197:84–92
Giannecchini R, D’Amato Avanzi G (2012) Historical research as a tool in estimating the flood/landslide hazard in a typical small alpine-like area: the example of the Versilia River basin (Apuan Alps, Italy). J Phys Chem Earth 49:32–43
Giannecchini R, Petrini R, D'Orazio M, Molli G, Vezzoni S, Perotti M, Cinquini I, Ghezzi L, Biagioni C, Di Giuseppe G, Fusi C, Vittori Antisari L, Vianello G, Doveri M, Guidi M, Menichini M, Baneschi I, Lelli M, Stenni B (2016) Studio multidisciplinare integrato (geologico-ambientale) nel bacino del Torrente Baccatoio. http://www.comune.pietrasanta.lu.it/allegati/44/UNIPI_Relazione_Finale_14-06-2016.pdf. Accessed 18 June 2021
Gismera MJ, Lacal J, da Silva P, García R, Sevilla MT, Procopio JR (2004) Study of metal fractionation in river sediments. A comparison between kinetic and sequential extraction procedures. Environ Pollut 127:175–182
Godt J, Scheidig F, Grosse-Siestrup C et al (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. https://doi.org/10.1186/1745-6673-1-22
Grolimund D, Borkovec M, Barmettler K, Sticher H (1996) Colloid-facilitated transport of strongly sorbing contaminants in natural porous media: a laboratory column study. Environ Sci Technol 30:3118–3123
Gruszecka-Kosowska A, Baran P, Wdowin M, Franus W (2017) Waste dolomite powder as an adsorbent of Cd, Pb(II), and Zn from aqueous solutions. Environ Earth Sci 76:521. https://doi.org/10.1007/s12665-017-6854-8
Guéguen C, Dominik J (2003) Partitioning of trace metals between particulate, colloidal and truly dissolved fractions in a polluted river: the upper Vistula River (Poland). Appl Geochem 18:457–470
Gunnars A, Blomqvist S, Johansson P, Anderson C (2002) Formation of Fe(III) oxyhydroxides colloids in freshwater and brackish seawater, with incorporation of phosphate and calcium. Geochim Cosmochim Acta 66:745–758
Han Y-S, Youm S-J, Oh C, Cho Y-C, Ahn JS (2017) Geochemical and eco-toxicological characteristics of stream water and its sediments affected by acid mine drainage. CATENA 148:52–59
Harvey JW, Bencala KE (1993) The effects of streambed topography on surface-subsurface water exchange in mountain catchments. Water Resour Res 29:89–98
Jung HB, Zheng Y, Rahman MW, Rahman MM, Ahmed KM (2015) Redox zonation and oscillation in the hyporheic zone of the Ganges-Brahmaputra-Meghna delta: implications for the fate of groundwater arsenic during discharge. Appl Geochem 63:647–660
Lattanzi P, Benvenuti M, Costagliola P, Tanelli G (1994) An overview on recent research on the metallogeny of Tuscany, with special reference to Apuane Alps. Mem Soc Geol Ital 48:613–625
Lee G, Faure G, Bigham JM, Williams DJ (2008) Metal release from bottom sediments of Ocoee Lake no. 3, a primary catchment area for the Ducktown mining district. J Env Qual 37:344–352
Lee H, Kim D, Kim J, Ji M-K, Han Y-S, Park Y-T, Yun H-S, Choi J (2015) As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite. J Hazard Mater 292:146–154
Martin-Fernandez JA, Hron K, Templ M, Filzmoser P, Palarea-Albaladejo J (2015) Bayesian-multiplicative treatment of count zeros in compositional data sets. Stat Modelling 15(2):134–158
McCluskey AH, Grant SB, Stewardson MJ (2016) Flipping the thin film model: Mass transfer by hyporheic exchanhe in gaining and losing streams. Water Resour Res 52:7806–7818
McKenzie RM (1980) The adsorption of lead and other heavy metals on oxides of manganese and iron. Ast J Soil Res 18:61–73
Molli G, Giorgetti G, Meccheri M (2002) Tectono-metamorphic evolution of the Alpi Apuane metamorphic complex: new data and constraints for geodynamic models. Boll Soc Ital 121:789–800
Natali S, Franceschi L, Doveri M, Giannecchini R (2019) Hydrogeological, chemical and water isotopes survey to characterize the groundwater flow system of Moresco springs (Apuan Alps - NW Tuscany). Rend Online Soc Geol It 48:17–22
Nordstrom DK, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. Appl Geochem 57:3–16
Owens PN, Batalla RJ, Collins AJ, Gomez B, Hicks DM, Horowitz AJ, Kondolf GM, Marden M, Page MJ, Peacock DH, Petticrew EL, Salomons W, Trustrum NA (2005) Fine-grained sediment in river systems: environmental significance and management issue. River Res Appl 21:693–717
Park JH, Han Y-S, Y-S, Ahn JS, (2016) Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res 106:295–303
Peretyazhko T, Zachara JM, Boily JF, Xia Y, Gassman PL, Arey BW, Burgos WD (2009) Mineralogical transformations controlling acid mine drainage chemistry. Chem Geol 262:169–178
Perotti M, Petrini R, D’Orazio M, Ghezzi L, Giannecchini R, Vezzoni S (2018) Thallium and other potentially toxic elements in the Baccatoio Stream catchment (northern Tuscany, Italy) receiving drainages from abandoned mines. Mine Water Environ 37:431–441
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385
Resongles E, Casiot C, Freydier R, Dezileau JV, Elbaz-Poulichet F (2014) Persisting impact of hystorical mining activity to metal (Pb, Zn, Cd, Tl, Hg) and metalloids (As, Sb) enrichment in sediments of the Gardon River, southern France. Sci Total Environ 481:509–521
Sánchez-España J (2007) The behavior of iron and aluminum in acid mine drainage: speciation, mineralogy, and environmental significance. In: Letcher TM (ed) Thermodynamics, solubility and environmental issues. Elsevier B.V., pp 137–150
Sawyer AH, Bayani Cardenas M, Buttles J (2011) Hyporheic exchange due to channel-spanning logs. Water Resour Res 47:W08502. https://doi.org/10.1029/2011WR010484
Schippers A, Jørgensen BB (2001) Oxidation of pyrite and iron sulfide by manganese dioxide in marine sediments. Geochim Cosmochim Acta 65:915–922
Singer DM, Jefferson AJ, Traub EL, Perdrial N (2018) Mineralogical and geochemical variation in stream sediments impacted by acid mine drainage is related to hydro-geomorphic setting. Elem Sci Anth 6:31. https://doi.org/10.1525/elementa.286
SkMd E, Trophathy S, Sahoo PK, Panigrahi MK (2013) Metal behavior in sediment associated with acid mine drainage stream: role of pH. J Geochem Explor 124:230–237
Smith KS, Ficklin WH, Plumlee GS, Meier AL (1992) Metal and arsenic partitioning between water and suspended sediment at mine-drainage sites in diverse geologic settings. In: Kharaka YK, Maest AS (eds) Water-Rock Interaction, Proc, 7th International Symp on Water-Rock Interaction, vol 1. Balkema, Rotterdam, pp 443–447
Smith KS, Plumlee GS, Ranville JF, Macalady DL (1996) Predictive double layer modeling of metal sorption in mine-drainage systems. In: Jenne EA (ed) Adsorption of metals by Geomedia: variable mechanisms and model applications. Academic Press, Washington
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley, New York
Sun Q, Cui PX, Liu C, Peng SM, Alves ME, Zhou DM, Shi ZQ, Wang YJ (2019) Antimony oxidation and sorption behavipur on birnessite with different properties (δ-MnO2 and triclinic birnessite). Environ Pollut 246:990–998
Triska FJ, Kennedy VC, Avanzino RJ, Zellweger GW, Bencala KE (1989) Retention and transport of nutrients in a third order stream in northwestern California: hyporheic processes. Ecology 70:1893–1905
Varmusa K, Filzmoser P (2009) Introduction to multivariate statistical analysis in chemometrics. CRC Press, Taylor & Francis Group
Vilgé-Ritter A, Rose J, Masion A, Bottero JY, Lainé JM (1999) Chemistry and structure of aggregates formed with Fe-salts and natural organic matter. Colloids Surf A 147:297–308
Vittori Antisari L, Bini C, Ferronato C, Gherardi M, Vianello G (2019) Translocation of potential toxic elements from soil to black cabbage (Brassica oleracea L.) growing in an abandoned mining district area of the Apuan Alps (Tuscany, Italy). Environ Geochem Health. https://doi.org/10.1007/s10653-019-00443-y
Weber FA, Hofacker AF, Voegelin A, Kretzschmar R (2010) Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil. Environ Sci Technol 44:116–122
Weber J, Bracco JN, Poplawsky JD, Levlev AV, More KL, Lorenz M, Bertagni AL, Jindra SA, Starchenko V, Higgins SR, Stack AG (2018) Unraveling the effects of strontiumiIncorporation on baryte growth-in situ and ex situ observations using multiscale chemical imaging. Cryst Growth Des 18:5521–5533
Xie Y, Lu G, Yang C, Qu L, Chen M, Guo C, Dang Z (2018) Mineralogical characteristics of sediments and heavy metal mobilization along a river watershed affected by acid mine drainage. PLoS ONE 13(1):e0190010
Yu J-Y, Heo B, Choi I-K, Cho J-P, Chang H-W (1999) Apparent solubilities of schwertmannite and ferrihydrite in natural stream waters polluted by mine drainage. Geochim Cosmochim Acta 63:3407–3416
Acknowledgements
M. D’Orazio performed ICP-MS and HHXRF analysis on the sediments and is sincerely acknowledged. I. Baneschi helped during sediment coring. C. Biagioni is thanked for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. 1S
Concentrations (mg/kg) of a Fe, Al, Mn, Ni, Cu, Zn, As, and Cd, and b Sb, Tl, Pb, Sr, and Ba in bank-full channel sediment samples, ordered from the headwater downstream stations. The distance from head source is also labelled in (b). Solid line: 0–20 cm depth; dashed line: 40–80 cm depth (PPTX 72 KB)
Fig. 2S
Fe, As, Sr, Ba, S, Ni, Mn, Sb (a) and Pb, Zn, Ca, K (b) concentration (mg/kg) profile in streambed sediment core (PPTX 57 KB)
Fig. 3S
a Fe(II) oxidation rate and b Fe(II) concentration pattern in the Baccatoio stream water increasing distance from Pollone AMD input to station C7-2 (PPTX 42 KB)
Rights and permissions
About this article
Cite this article
Ghezzi, L., Buccianti, A., Giannecchini, R. et al. Geochemistry of Mine Stream Sediments and the Control on Potentially Toxic Element Migration: A Case Study from the Baccatoio Basin (Tuscany, Italy). Mine Water Environ 40, 722–735 (2021). https://doi.org/10.1007/s10230-021-00789-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-021-00789-9