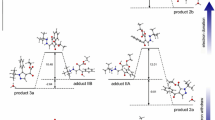
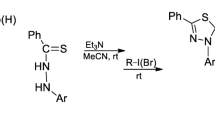
In this work, new pyrazole derivatives were prepared by one-pot three-component reaction emloying symmetric and asymmetric 1,2-disulfonyl-hydrazines, cyclohexyl isocyanide, and dialkyl acetylenedicarboxylates. Reactions with asymmetric 1,2-disulfonylhydrazines led to two regioisomers, which were distinguished by density functional theory calculations and the major isomer attributed to the energically more stable one.


Similar content being viewed by others
References
(a) Evano, G.; Blanchard, N.; Toumi, M. Chem. Rev. 2008, 108, 3054. (b) Patil, N. T.; Yamamoto, Y. Chem. Rev. 2008, 108, 3395. (c) Whitehouse, M. W. Curr. Med. Chem. 2005, 12, 2931. (d) Yet, L. Chem. Rev. 2000, 100, 2963. (e) Zhou, Q. F.; Ge, F. F.; Chen, Q. Q.; Lu, T. RSC Adv. 2016, 6, 1395.
Alam, M. J.; Alam, O.; Alam, P.; Naim, M. J. Int. J. Pharma Sci. Res. 2015, 6, 1433.
Nimbalkar, S.; Hote, S. V. ICRITCC 2015, 3, 61.
Mathew, A.; Sheeja, M.; Kumar, A.; Radha, K. Hygeia: J. Drugs Med. 2011, 3(2), 48.
Hamri, S.; Rhazri, K.; Hafid, A.; Ouchetto, H.; Hajbi, Y.; Khouili, M. Global J. Sci. Fro. Res. 2013, 13(7-B), 1.
Dawane, B. S.; Konda, S. G.; Shaikh, B. M.; Chobe, S. S.; Khandare, N. T.; Kamble, V. T.; Bhosale, R. B. Int. J. Pharm. Sci. Rev. Res. 2010, 44.
Selvam, T. P.; Kumar, P. V.; Saravanan, G.; Prakash, C. R. J. Saudi Chem. Soc. 2014, 18, 1015.
Kumar, K. A.; Jayaroopa, P. Int. J. PharmTech Res. 2013, 5, 1473.
(a) Wu, J.; Song, B.-A.; Hu, D.-Y.; Yue, M.; Yang, S. Pest Manag. Sci. 2012, 68, 801. (b) Jiang, D.; Zheng, X.; Shao, G.; Ling, Z.; Xu, H. J. Agric. Food Chem. 2014, 62, 3577. (c) Cole, L. M.; Nicholson, R. A.; Casida, J. E. Pestic. Biochem. Phys. 1993, 46, 47.
(a) Abid, M.; Bhat, A. R.; Athar, F.; Azam, A. Eur. J. Med. Chem. 2009, 44, 417. (b) Bhat, A. R.; Athar, F.; Azam, A. Eur. J. Med. Chem. 2009, 44, 426. (c) Ciupa, A.; De Bank, P. A.; Mahon, M. F.; Wood, P. J.; Caggiano, L. MedChemComm 2013, 4, 956. (d) Palaska, E.; Aytemir, M.; Uzbay, I. T.; Erol, D. Eur. J. Med. Chem. 2001, 36, 539. (e) Palaska, E.; Erol, D.; Demirdamar, R. Eur. J. Med. Chem. 1996, 31, 43. (f) Prasad, Y. R.; Rao, A. L.; Prasoona, L.; Murali, K.; Kumar, P. R. Bioorg. Med. Chem. Lett. 2005, 15, 5030. (g) Yang, C.; Li, J.; Zhou, R.; Chen, X.; Gao, Y.; He, Z. Org. Biomol. Chem. 2015, 13, 4869.
(a) de los Santos, J. M.; Lopez, Y.; Aparicio, D.; Palacios, F. J. Org. Chem. 2008, 73, 550. (b) Fache, F.; Schulz, E.; Tommasino, M. L.; Lemaire, M. Chem. Rev. 2000, 100, 2159. (c) Guerra, F.; Mish, M.; Carreira, E. Org. Lett. 2000, 2, 4265.
Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications; Wiley-VCH Verlag: Weinheim, 2013, p. 236.
(a) Chen, J. R.; Dong, W. R.; Candy, M.; Pan, F. F.; Jörres, C.; Bolm, M. J. Am. Chem. Soc. 2012, 134, 6924. (b) Cui, S.-L.; Wang, J.; Wang, Y.-G. Org. Lett. 2008, 10, 13. (c) Kanemasa, S.; Kanai, T. J. Am. Chem. Soc. 2000, 122, 10710. (d) Kissane, M.; Maguire, A. R. Chem. Soc. Rev. 2010, 39, 845. (e) Nair, V.; Biju, A.; Mohanan, K.; Suresh, E. Org. Lett. 2006, 8, 2213. (f) Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484. (g) Suárez, A.; Downey, C. W.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 11244. (h) Wang, Y.; Rivera Vera, C. I.; Lin, Q. Org. Lett. 2007, 9, 4155. (i) Yang, C.; Liu, W.; He, Z.; He, Z. Org. Lett. 2016, 18, 4936. (j) Zhang, D. Y.; Shao, L.; Xu, J.; Hu, X. P. ACS Catal. 2015, 5, 5026.
(a) Alex, K.; Tillack, A.; Schwarz, N.; Beller, M. Org. Lett. 2008, 10, 2377. (b) Patil, N. T.; Singh, V. Chem. Commun. 2011, 11116.
Müller, S.; List, B. Angew. Chem., Int. Ed. 2009, 48, 9975.
(a) Wei, Q.; Chen, J. R.; Hu, X. Q.; Yang, X. C.; Lu, B.; Xiao, W. J. Org. Lett. 2015, 17, 4464. (b) Duan, X. Y.; Yang, X. L.; Fang, R.; Peng, X. X.; Yu, W.; Han, B. J. Org. Chem. 2013, 78, 10692.
Zhang, Z.; Wang, D.; Wei, Y.; Shi, M. Chem. Commun. 2012, 9607.
(a) Soltanzadeh, Z.; Imanzadeh, G.; Noroozi-Pesyan, N.; Şahin, E.; Hooshmand, H. Tetrahedron 2016, 72, 1736. (b) Largani, T. H.; Imanzadeh, G.; Noroozi Pesyan, N.; Şahin, E.; Shamkhali, A. N.; Notash, B. Mol. Diversity 2018, 22, 37.
Soltanzadeh, Z.; Imanzadeh, G.; Noroozi-Pesyan, N.; Şahin, E. Green Chem. Lett. Rev. 2017, 10, 148.
(a) Zhao, Y.; Truhlar, D. G. J. Phys. Chem., A 2006, 110, 5121. (b) Francl, M. M.; Pietro, W. J.; Hehre, W. J.; Binkley, J. S.; Gordon, M. S.; DeFrees, D. J.; Pople, J. A. J. Chem. Phys. 1982, 77, 3654.
Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347.
Bartmann, E. A. Synthesis 1993, 490.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Authors are grateful to the University of Mohaghegh Ardabili for the financial support and the laboratories of Tehran University and Tabriz University for the product analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(6), 640–645
Supplementary Information
ESM 1
(PDF 14189 kb)
Rights and permissions
About this article
Cite this article
Safari, N., Imanzadeh, G., Asgharzadeh, R. et al. Highly functionalized pyrazolines from symmetric and asymmetric 1,2-disulfonylhydrazines: a combination of experimental and DFT perspectives. Chem Heterocycl Comp 57, 640–645 (2021). https://doi.org/10.1007/s10593-021-02962-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02962-y