Abstract
Purpose
Pathogenic variants in Lysyl-tRNA synthetase 1 (KARS1) have increasingly been recognized as a cause of early-onset complex neurological phenotypes. To advance the timely diagnosis of KARS1-related disorders, we sought to delineate its phenotype and generate a disease model to understand its function in vivo.
Methods
Through international collaboration, we identified 22 affected individuals from 16 unrelated families harboring biallelic likely pathogenic or pathogenic in KARS1 variants. Sequencing approaches ranged from disease-specific panels to genome sequencing. We generated loss-of-function alleles in zebrafish.
Results
We identify ten new and four known biallelic missense variants in KARS1 presenting with a moderate-to-severe developmental delay, progressive neurological and neurosensory abnormalities, and variable white matter involvement. We describe novel KARS1-associated signs such as autism, hyperactive behavior, pontine hypoplasia, and cerebellar atrophy with prevalent vermian involvement. Loss of kars1 leads to upregulation of p53, tissue-specific apoptosis, and downregulation of neurodevelopmental related genes, recapitulating key tissue-specific disease phenotypes of patients. Inhibition of p53 rescued several defects of kars1−/− knockouts.
Conclusion
Our work delineates the clinical spectrum associated with KARS1 defects and provides a novel animal model for KARS1-related human diseases revealing p53 signaling components as potential therapeutic targets.
Similar content being viewed by others
INTRODUCTION
Aminoacyl-tRNA synthetases (ARSs) are ubiquitously expressed and essential enzymes required for the aminoacylation of specific amino acids onto their cognate transfer RNAs (tRNAs). Biallelic variants in ARS genes have been shown to cause a variety of severe and early-onset human diseases [1]. These diseases appear with diverse clinical manifestations such as Charcot–Marie–Tooth disease [2], leukodystrophies [1, 3,4,5], cardiomyopathies [6], hearing loss, [7] and other central nervous system (CNS)–related pathologies [8,9,10,11]. ARS variants can lead to reduced aminoacylation activity, decreased translation accuracy, and defects in noncanonical processes [1], but the underlying mechanisms leading to pathology remain poorly understood [12]. As a result, there are no effective treatment strategies for these pathologies.
Of the 37 ARS genes in humans, 18 encode cytoplasmic enzymes, 17 encode mitochondrial enzymes, and 2 encode bifunctional enzymes; KARS1 (lysyl-tRNA synthetase) is one of the bifunctional ARS enzymes. In humans, a single KARS1 gene (MIM 601421) [13] encodes both the cytoplasmic and mitochondrial lysyl-tRNA synthetases, which are generated by alternative splicing. Biallelic variants in KARS1 have been reported in patients with a broad spectrum of clinical manifestations including Charcot–Marie–Tooth [2], nonsyndromic hearing loss [14, 15], peripheral neuropathy [2], congenital visual impairment [16], progressive microcephaly [16], hypertrophic cardiomyopathy [17], leukoencephalopathies [15, 18, 19], leukodystrophy, [20] and severe neurological and neurosensory disease with optic neuropathy [21]. Twenty-eight pathogenic variants in 30 affected individuals from 25 families have been identified to date, following an autosomal recessive inheritance pattern.
Here, we report 10 new and 4 known biallelic missense variants in KARS1 in 22 affected individuals from 16 unrelated families. Having included data from 30 previously published KARS1 cases, we provide a cumulative and comprehensive phenotypic characterization of 52 affected individuals. A mouse knockout of Kars1 is embryonic lethal and dies before organogenesis occurs [22]. Nevertheless, the function of Kars1 in specific tissues in vivo remained unknown. Here, we generated loss-of-function alleles in zebrafish using CRISPR/Cas9 to understand the function of kars1 in vivo.
MATERIALS AND METHODS
Genetic and phenotypic analysis
Blood samples were collected from all participants and genomic DNA was extracted using standard methods. Sequencing methods and genetic analysis summaries per individual sequenced in each family are summarized in Table S1. Clinical information was collected using standardized templates and completed by collaborating geneticists and clinicians. Brain magnetic resonance images (MRIs)/computed tomography (CT) images from all previously published reports and our cohort were systematically analyzed by a pediatric neuroradiologist. Detailed clinical methods are described in Supplementary information.
Zebrafish functional studies
Zebrafish (Danio rerio) were raised and maintained in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility at the Oklahoma Medical Research Foundation (OMRF) under standard conditions, and all experiments were performed as per protocol 17-01 approved by the Institutional Animal Care Committee (IACUC) of OMRF. All zebrafish work was carried out in wild-type (WT) strain NHGRI-1. Detailed methods related to zebrafish work are described in Supplementary information.
Statistical analysis
Each experiment was repeated three times, and sample sizes are described in the Supplementary information. Data are presented as mean value ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA, USA). In all analyses, the significance level was set to 0.05. The p value was determined as follows. The larval survival curve (Kaplan–Meier representation) was assessed using the log rank Mantel–Cox test. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used for eye and head size comparisons. Two-tailed unpaired Student’s t-test with nonparametric Mann–Whitney test was used for hair cell and motor exon diameter calculation. Two-tailed unpaired Student’s t-test was used for the comparison of gene expression levels between two groups, and Holm–Šídák multiple comparisons correction was used for for multiple groups comparison. Two-tailed unpaired t-test with Welch’s correction was used for the visual startle response (VSR) and auditory evoked behavior response (AEBR) analyses.
RESULTS
Patients with KARS1 variants show multisystem abnormalities primarily involving the nervous system
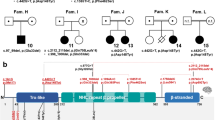
We investigated 22 affected individuals from 16 unrelated families who harbored biallelic KARS1 variants (Fig. 1a). Clinical summaries and variant details are presented in the Case Reports in Supplementary information, Table 1, Fig. 1c, and Tables S2 and S3. All patients underwent either exome, genome, or targeted gene sequencing and analyses, which excluded other functionally relevant variants compatible with Mendelian diseases, based on mode of inheritance and clinical presentation. Variants were Sanger sequence–confirmed (Fig. S1). We identified ten previously unpublished variants and four previously reported variants [18, 19, 21, 23, 24] as listed in Table 1, and Fig. S2. Each substitution affected a conserved amino acid, and all the variants are predicted to be deleterious (Supplementary information, Fig. S3).
(a) Pedigrees and segregation data for the 16 families included in this study. Affected and unaffected individuals are indicated by filled and open squares (males) and circles (females), respectively. Probands are marked with red arrows. Double lines indicate consanguinity. Genetic diagnoses were made in 22 individuals. (b) Clinical characteristics of patients with homozygous KARS1 variants, including affected individuals from family 1 (IV:4) (i, ii), family 2 (IV:3) (iii), family 4 (V:3) (iv) and (V:4) (v), family 5 (II:1) (vi), family 7 (IV:1) (vii, viii) and IV:4 (ix), and family 8 (IV:1) (x) (IV:2) (xi). Frequent clinical features include: spasticity and contractures in the limbs with clenched hands (i, ii), high forehead (iv, v, vi, vii, x), prominent nose (i, ii, iv, v, vi, vii, viii), low-set ears (i, ii, iv, v, vii, viii, ix), and short philtrum (i, ii, iv, v, vi, vii, ix, x). (c) Phenotype summary of features associated with KARS1 pathogenic variants. Asterisk denotes movement disorders that include ataxia, spasticity, quadriplegia, dystonia, or chorea/tremor. (d–o) Neuroimaging features associated with KARS1 variants, with variable patterns of white matter (WM) involvement (red arrows), calcifications (red dotted arrows), pontine hypoplasia (red arrowheads), cerebellar atrophy (red thick arrows), enlargement of the cerebral cerebrospinal fluid (CSF) spaces, and corpus callosum hypoplasia (gold arrows).
Clinical description
The cohort mostly presented with a moderate-to-severe developmental delay, progressive neurological and neurosensory impairment, and white matter involvement, variably associated with developmental regression, intellectual disability, behavioral abnormalities, and additional extraneurological signs. The disease typically manifested during early childhood with developmental delay and/or hearing impairment. Developmental regression was reported in 7/22 cases (32%). Four cases died at the mean age of 13.9 years due to respiratory infection (2/4), sepsis (1/4), or postfebrile illness associated with severe deterioration (1/4). All parents were asymptomatic and did not exhibit neurological symptoms. Developmental delay was present in 86% of cases (19/22) followed by intellectual disability (15/21, 71%), speech delay (11/19, 58%), absent speech (11/20, 55%), failure to thrive (4/22, 18%), and behavioral abnormalities such as autism (3/21, 14%) and hyperactive behavior (6/21, 29%).
All cases uniformly expressed neurological symptoms (22/22, 100%), frequently involving sensorineural hearing loss (20/21, 95%), seizures (13/22, 59%), hypotonia (9/22, 41%), cerebellar ataxia (7/22, 32%), spasticity (8/22, 36%), strabismus (6/20, 30%) and nystagmus (4/20, 20%), and acquired joint contractures (4/22,18%). Hearing loss was severe-to-profound in 16/21 cases (76%), and 5/10 (50%) of them had cochlear implants. Other variable neurological features included visual impairment/optic atrophy (7/20, 35%), quadriplegia (3/22, 14%), dystonia and tremor (2/22, 9%), neuropathy (1/10, 10%), neurophysiologically confirmed skeletal myopathy (2/22, 9%) (Video S1-2), generalized muscle atrophy (2/22, 9%), and incontinence (4/22, 18%). None of the cases expressed isolated hearing loss or sensory–motor neuropathy as the main clinical feature.
While not every case was uniformly examined, roughly half were clinically diagnosed with microcephaly (10/18, 56%). Interestingly, dysmorphic facial features were common in 67% (14/21) of the cohort and this included a high or narrow forehead, prominent nose, short philtrum, low-set ears, broad nasal bridge, thin upper lip, epicanthus, and telecanthus (Fig. 1b; Supplementary Case Report includes detailed descriptions). Other extraneurological signs included feeding difficulties (6/22, 27%), and neonatal vomiting with diarrhea (2/22, 11%). Single isolated cases displayed hypertrophic cardiomyopathy (1/11, 9%), and neonatal vomiting with diarrhea (2/22, 11%). The case with hypertrophic cardiomyopathy did not have available levels of lactate and mitochondrial respiratory chain enzymes measured.
Neuroimaging findings
Brain MRI studies were available for 21/22 (95%) individuals (with more than one examination in 2 cases), while head CT and spinal MRI were available for three and one subjects, respectively (Fig. 1d–o). White matter involvement was noted in 13/21 (61.9%) individuals. In 5/13 (38.4%) subjects, there were confluent T2 hyperintensities in the periventricular white matter, with prevalent involvement of parieto-occipital regions; one of these subjects studied at 53 years of age had a previous normal brain MRI (performed 8 years before). In 4/13 (23%) subjects, there was mild diffuse T2 hyperintensity of the supratentorial white matter, with sparing of the corpus callosum and U fibers; one of these individuals studied at 4 years of age had a previous normal brain MRI (performed 2 years before). Two subjects (2/13, 15.3%) presented diffuse leukodystrophy with corpus callosum involvement, sparing of the U fibers, and calcifications; in one of them the cerebellar white matter was also involved. Delayed myelination was noted in 2 other subjects (2/13, 15.3%). White matter volume loss with ventricular enlargement was present in 10/21 (47.6%) cases. Enlargement of the cerebral CSF spaces, mainly in the frontotemporal regions was observed in 9/21 (42.8%) individuals. Hypoplasia of the pons and corpus callosum was noted in 7/21 (33.3%) and 6/21 (28.5%) of cases, respectively. Cerebellar atrophy, with prevalent or isolated vermian involvement was noted in 5/21 (23.8%) of cases. Finally, in 4/21 (19%) subjects, the brain MRI was normal.
Genotype–phenotype correlation
Inter- and intrafamilial phenotypic variability was noticed among individuals with the same variants in the present KARS1 cohort. For instance, three affected siblings in family 11 each harbored a homozygous KARS1 c.379T>C (p.[Phe127Leu]) variant, and although they shared common symptoms such as developmental delay, infantile-onset profound hearing loss, dysmorphic facial features, spasticity, and varying degree of joint contractures, several important symptoms were expressed only by one of them. This included regression, epilepsy, optic atrophy, failure to thrive, and hyperactivity. The same KARS1 variant was homozygous in the proband from family 1, who differed from family 11 by a preserved vision, cerebellar ataxia, classic leukodystrophy on MRI, and the basal ganglia calcification. Similarly, five affected members from three independent families were homozygous for the KARS1 c.1772A>T (p.[Asn591Ile]) variant and they had variable intra- and interfamilial expression of epileptic seizures, cerebellar ataxia, intellectual disability, hypotonia, polyneuropathy, behavioral abnormalities, and impaired speech. Significant phenotypic variability was also noticed between families 9 and 15 harboring the similar compound heterozygous KARS1 variants c.683C>T (p.[Pro228Leu]) and c.774A>T (p.[Arg258Ser]).
kars1 zebrafish disease model using CRISPR/Cas9-mediated targeted mutagenesis
Zebrafish has a single kars1 gene (NCBI Gene ID: 280647) generating two transcript variants via alternative RNA splicing, confirmed by reverse transcription polymerase chain reaction (RT-PCR) from multiple developmental stages (Fig. S4, and Supplementary information). To unravel the function of KARS1, we examined kars1 messenger RNA (mRNA) expression in zebrafish embryonic development using whole-mount in situ hybridization (WISH). kars1 mRNA was initially ubiquitously expressed but gradually became more prominent in the CNS, eye, inner ear, muscles, and digestive system (liver, intestine, and pancreas) (Fig. 2a–c, Supplementary information, and Fig. S5a–g). We generated kars1 loss-of-function zebrafish mutant lines using CRISPR/Cas9 and identified three independent alleles kars1om1del7, kars1om2del8, and kars1om3del7 (Fig. S6a–d). Homozygous kars1−/− animals showed morphological abnormalities starting at approximately 3 dpf including heart edema (black arrow), as well as smaller heads (blue line), eye (red line) and otic vesicle (red arrowhead) when compared to WT animals (Fig. 2d, e, and Fig. S7a–c). Kars1 homozygous larvae also failed to inflate the swim bladder (black arrowhead) and showed abnormal trunk muscle fibers. We observed 100% mortality by 10 dpf for all three kars1 homozygous mutant larvae, possibly because of the inability to feed (Fig. 2f and Fig. S7d). We quantified the eye and head axial length which were significantly reduced in kars1−/− mutants compared to control animals (Fig. 2g, h). Additionally, the kars1−/− larvae failed to respond to touch and displayed a loss of spatial orientation (Fig. S7e, f and Video S3). The side-laying position observed in kars1−/− mutant larvae might reflect loss of vestibular function, severe muscle control failure, and/or absence of an inflated swim bladder. which all affect balance. Given the kars1−/− mutants showed morphological defects in the eyes and ears, and failed to response to touch, we further quantified their VSR and AEBR; kars1−/− mutants showed completed loss of locomotor activity in response to light or acoustic startle (Fig. 2I, j). Overall, the tissue/organ-specific morphological defects in kars1−/− mutants found in the eye, inner ear, and trunk muscles appeared to directly correlate with kars1 mRNA expression patterns during embryo development, strongly suggesting these phenotypes and behaviors arose from Kars1 loss of function.
(a) kars1 expression in 1 days postfertilization (dpf) embryo. Dorsal view. (b,c) The cross-section of 2 dpf embryo. CMZ ciliary marginal zone, fb forebrain, hb hindbrain, le lens, mb midbrain, MHB midbrain and hindbrain boundary, ov otic vesicle, TeO optic tectum. Black arrowheads indicating the otic vesicle epithelium of otic vesicles. (d,e) Representative images of wild-type (WT) and kars1om1del7 (kars1−/−) from 1 dpf to 5 dpf. Lateral view, anterior to the left. Red lines: eye diameter. Blue lines: brain size. Red arrowheads: inner ear. Black arrows: heart edema. Black arrowhead: swim bladder. (f) Kaplan–Meier survival curves. Time is shown in days. The log rank test was used for statistical analysis. (g,h) Quantification of eye and head size from kars1+/- mutant in-cross at 3 and 5 dpf. (i,j) The visual startle response (VSR) and auditory evoked behavior response (AEBR) analyses of animals at 6 dpf from kars1+/- mutant in-cross. (k) Representative images of kars1−/− mutant rescue experiments and the quantification of heart edema phenotype. Animals were collected by defined heart edema categories at 3 dpf as shown in pictures and calculated in percentage of total animals. (l) Eye size quantification of mRNA rescue experiments at 5 dpf. (m,n) The VSR and AEBR analyses after RNA rescue at 6 dpf. n = number of animals. In (g,h,l), each dot represents one animal and error bars are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test: ****p < 0.0001. In (i,j,m,n), data are plotted by box and whiskers plot and error bars indicate values from minimum to the maximum. Two-tailed unpaired t-test with Welch’s correction: ns, not significant p ≥ 0.05, *p < 0.05 and ****p < 0.0001.
To confirm the phenotypes of the kars1−/− mutant animals arise from loss of kars1 function, we performed mRNA rescue experiments by injecting either human WT KARS1 or the zebrafish WT kars1 mRNA. Coinjection of mRNAs encoding the mitochondrial and cytoplasmic isoforms into 1-cell stage showed reduced frequencies of heart edema (Fig. 2k) and significantly restored eye size in kars1−/− mutants (Fig. 2l). Furthermore, microinjection of zebrafish kars1 mRNA further rescued the startle responses compared to kars1−/− mutant animals (Fig. 2m, n) suggesting mutant phenotypes are caused by kars1 loss of function.
The kars1 loss-of-function zebrafish model recapitulates patient symptoms
Histological analysis of the brain of 5-dpf larvae revealed a vacuolated spongiosus appearance with areas of reduced cell density and disorganized segment boundaries in kars1−/− mutants compared to WT siblings (Fig. 3a–c). Moreover, eye volume was reduced and the retinal layer organization was completely lost in the kars1−/− mutants compared to WT siblings (Fig. 3b) strongly suggesting impaired vision. Furthermore, the number of neuronal cells appeared strongly reduced in the brain and retina in kars1−/− mutants compared to controls (Fig. 3a, b). Immunohistochemical analysis of neuronal synapses revealed reduced staining in all brain and eye regions pointing to a significant reduction of synapses in kars1−/− mutants (Fig. 3d), suggesting impaired neuronal transmission. Additionally, staining revealed abnormal motor neuron morphology, including shrinkage of motor neuron axon projections and reduction of terminal axonal branching in the mutants (Fig. 3e), further indicating strong alteration of locomotor function in those animals.
(a) Head region of wild-type (WT) and kars1−/− mutant by sagittal section. Red asterisks indicate massive loss of cell density, as well as in (b) and (c). Anterior to the left and dorsal to the top. (b) Eye region of WT and kars1−/− mutant by cross-section. Dorsal to the top. (c) Inner ear region of WT and kars1−/− mutant by cross-section. Dorsal to the top. Lower panels were enlarged picture from black box. (d) Head region of WT and kars1−/− larvae immunostained with anti-znp-1 antibody. The black-and-white fluorescent signals were inverted to negative film for a clear presentation. Dorsal view, anterior to the left. on, optic nerve. (e) Trunk region of WT and kars1−/− larvae which were immunostained with anti-znp-1 antibody. ImageJ was used to measure the diameter of primary motor axons as indicated by red line in the right-down panels (enlarged from red box). Statistics are shown on the right-hand side. Black dot indicates the diameter of each motor axon. Error bars = mean ± SD. Two-tailed unpaired nonparametric Mann–Whitney test: ****p < 0.0001. Black arrowheads indicate the reduced terminal axonal branching compared to WT. Anterior to the left and dorsal to the top. (f) The trunk region of WT and kars1−/− larvae revealed by sagittal section. Anterior to the left and dorsal to the top. (g) The trunk region of WT and kars1−/− larvae revealed by confocal projections of phalloidin stained muscle fiber. Anterior to the left and dorsal to the top. The lower-right panels are the higher-magnification view. (h) Representative bright-field images of WT and kars1−/− inner ear. Anterior to the left and dorsal to the top. oto otolith, SC semicircular canal. (i) The red fluorescent conjugated phalloidin staining was performed to visualize the bundles (stereocilia) of hair cells in inner ear. Anterior to the left and dorsal to the top. ac anterior crista, am anterior macula, ce cerebellum, GCL ganglion cell layer, hb hindbrain, INL inner nuclear layer, IPL inner plexiform layer, lc lateral crista, ONL outer nuclear layer, pc posterior crista.pm posterior macula, RPE retinal pigment epithelium, TeO optic tectum.
Individuals with biallelic KARS1 pathogenic variants usually present with combined neuronal and muscular dysfunction. Indeed, our WISH data revealed kars1 mRNA expression in trunk muscles (Fig. 2a) and a loss of touch-evoked responses in the kars1−/− mutants (Fig. S7e and Video S3). Phalloidin staining that labels actins showed weaker staining in the myotomes and presented misaligned and detached muscle fibers from the myotendinous junction (MTJ) (Fig. 3f, g) suggesting severe neuromuscular dysfunction leading to absence of coordinated locomotion.
Several individuals with KARS1 variant alleles also display hearing disorders reminiscent of the inner ear defects observed in kars1−/− mutant larvae, such as perturbed sensory epithelia and loss of AEBR startle response. Morphological analysis of the mutants showed smaller otic vesicles and otoliths (Fig. 3h) and flattened sensory epithelia in both anterior and posterior maculae (Fig. 3c). The phalloidin staining in the inner ear showed strong reduction in the number of F-actin-rich stereocilia, which are crucial for the formation of hair cell bundles and thus for mechanoelectrical transduction (Fig. 3i). Vital stain Yo-PRO-1 iodide, showed fewer hair cells in the zebrafish lateral line which are morphologically and functionally similar to those of the inner ear [25] (Fig. S8a, b). Taken together, these findings suggest that kars1 plays a crucial role in hair cell formation in zebrafish, reminiscent of hearing disorders found in a subset of individuals affected with KARS1 variants.
Variants in KARS1 have been shown to be associated with seizures (Table S2). Seizure activity positively correlates with the expression of c-fos (fosab in zebrafish), a marker for general neuronal activity. We used classical pro-chemoconvulsant drug pentylenetetrazol (PTZ) to induce seizure activity and elevated expression of fosab mRNA (Fig. S8c). Intriguingly, untreated kars1−/− larvae, when compared with untreated WT controls, exhibited twofold at 3 dpf and tenfold increase at 6 dpf in fosab mRNA levels (Fig. S8d) suggesting seizure-like activity in homozygous animals.
Together, our morphological, behavioral, and histological analyses demonstrate that the kars1−/− zebrafish mutant larvae exhibit visual impairment, neuromuscular dysfunction, sensorineural hearing loss, and increased expression of seizure marker, c-fos, thus recapitulating a number of pathologies found in individuals with KARS1 variants.
Kars1 loss of function triggers p53-mediated apoptosis and downregulation of key neurodevelopmental related genes
To determine the consequences of Kars1 loss of function on gene expression, we performed RNA-sequencing (RNA-Seq) on WT and kars1−/− mutants at 3 dpf and 4 dpf and compared gene expression profiles. We found 1,616 and 1,409 differentially expressed genes (DEGs) at 3 dpf and 4 dpf respectively, 563 genes overlapped between 3 dpf and 4 dpf (Fig. S9a–c, and Table S5). KEGG pathway analysis showed kars1 loss of function dysregulated many pathways including the p53 and cell apoptosis pathway (Figs. S10–12). ARSs have been shown to regulate cell death pathways [26, 27]. To test whether the morphological defects found in kars1−/− mutants were due to abnormal cell apoptosis, we performed a TUNEL assay and observed an increase in TUNEL-positive cells in the brain, eye, trunk, and inner ear of kars1−/− mutants compared with sibling animals at 5 dpf (Fig. S13a, b), suggesting distinct cell types are particularly sensitive to loss of Kars1. Next, to determine whether increased cell apoptosis was mediated by p53 activation, we knocked down tp53 by microinjecting kars1−/− mutant animals with tp53 antisense morpholinos (MO) and subsequently performed the TUNEL assays at 3 dpf. Results showed a significant reduction in the TUNEL-positive signals in the brain, eye, ear, and trunk in the MO-injected kars1−/− mutant (Fig. 4a–c). Strikingly, reduction in eye size (Fig. 4d) and upregulation of several apoptosis markers were restored to WT levels (Fig. 4e), therefore confirming the p53 pathway was successfully knocked down, and furthermore implicating it in reduction of eye size. To further characterize the functional rescue at the molecular level after inhibiting the p53 pathway, we examined the expression of six genes we previously found to be downregulated in kars1−/− mutants (Fig. 4f). All six genes showed at least partially restored expression upon p53 knockdown in kars1−/− mutants. Taken together, the loss of Kars1 upregulated the p53 pathway thus leading to apoptosis thereby causing multiple phenotypic abnormalities.
(a–c) Representative images of sibling, kars1−/−, and kars1−/− + p53 MO at 3 dpf after TUNEL staining. Red circle indicates eye region. Scale bars = 100 µm. (d) The eye size measurements of sibling, kars1−/− and kars1−/− + p53 MO at 3 dpf. (e) The expression levels of p53 pathway genes were examined by reverse transcription quantitative polymerase chain reaction (RT-qPCR) after p53 MO injection. (f) Those downregulated genes in RNA-seq data were examined by RT-qPCR after p53 MO injection. For (e,f), the expression levels were normalized to 18 S housekeeping gene. Error bars = mean ± SD. Two-tailed unpaired Student’s t-test with Holm–Šídák multiple comparisons correction: ns, not significant p ≥ 0.05, *p < 0.05, **p < 0.01, and ***p < 0.001. (g) Representative images of uninjected control, kars1 F0 mutant, kars1;tp53 F0 mutant, and kars1 F0 mutant coinjected with kars1 messenger RNA (mRNA) at 5 dpf. Lateral view, anterior to the left. (h,i) Measurement of eye and head size in control, tp53 F0 mutant, kars1 F0 mutant, kars1;tp53 F0 mutant, and kars1 F0 mutant coinjected with 150 picogram (pg) and 200 pg of kars1 mRNA at 5 dpf. For (d,h,i), each symbol represents one animal. Error bars = mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test: ns, not significant p ≥ 0.05 and ****p < 0.0001. (j) Kaplan–Meier survival curves. Time is shown in days. Log rank test: ****p < 0.0001. (k) Histology analysis of uninjected control, kars1 F0 mutant and kars1;tp53 F0 mutant by cross-section.
To validate our findings, we used CRISPR/Cas9 technology to generate biallelic variants. Coinjecting WT kars1 mRNAs with the kars1 sgRNAs rescued these phenotypes, confirming they arise from Kars1 loss of function (Fig. 4g). Importantly, coinjection of tp53 sgRNAs with kars1 sgRNAs restored the eye and head size of the kars1 F0 mutants (Fig. 4g–i). Moreover, whereas 90% of kars1 F0 mutant animals died by 10 dpf, only 20% of animals coinjected with either kars1 mRNAs or tp53 sgRNAs died by 10 dpf (Fig. 4j). Histological analysis showed vacuolated spongiosus appearance in the brain was significantly restored in kars1;tp53 F0 mutants compared to kars1 F0 mutants, as was eye volume and retinal layer organization (Fig. 4k) and vision/hearing startle responses (Fig. S13c, d). At the molecular level, kars1 F0 mutants showed significantly reduced kars1 expression and increased tp53 expression compared to the uninjected control, whereas kars1;tp53 F0 mutants showed significantly reduced expression of both kars1 and tp53 (Fig. S13e). Additionally, kars1 F0 mutants showed increased casp8 and decreased mbpa, neurod1, and stxbp1a expression compared to controls that was restored in kars1;tp53 F0 mutants (Fig. S13f). Together, our results showed kars1 F0 mutants phenocopy the kars1−/− mutant, and p53 depletion mitigates the morphological, behavior, and molecular phenotypes due to loss of Kars1.
DISCUSSION
Deciphering how variants in different ARS genes cause diverse organ-specific phenotypes is crucial to inform therapeutic approaches for ARS-related disorders. We report 10 novel and 4 known variants in KARS1 from 22 patients belonging to 16 unrelated families. Having included the clinical and neuroimaging data from our cohort and the corresponding data from the previously published 30 KARS1 cases (25 families), we made a cumulative phenotypic characterization for 52 cases with biallelic KARS1 variants (Fig. 1c).
Notably, our cohort expands the phenotypic spectrum of KARS1 variants to include autism/hyperactivity. Additionally, we highlight a variety of KARS1-associated dysmorphic facial features, as facial dysmorphism has rarely been described in previous KARS1 reports. It is reported in 11/52 individuals, where ten cases were identified in our cohort and one case from McLaughlin et al. [2]. We also provide further supporting evidence for skeletal myopathy, a phenotype that has previously been reported in one case [17]. Our case with hypertrophic cardiomyopathy (family 4) seems to also highlight the importance of this phenotype as in the previous few reports [17, 28]. Finally, we highlight cerebellar ataxia might be a frequent KARS1-associated feature that was previously reported only in 3/30 (10%) cases but present in 32% (7/22) of our cohort. Our cumulative phenotypic analysis showed that similar to other ARS, KARS1 expresses predominant neurological and neurosensory phenotypes associated with facial dysmorphism [29].
The systematic analysis of all available neuroimaging data, including the present cases, suggests the spectrum of manifestations associated with KARS1 variants is wide, ranging from normal brain appearance to a severe leukodystrophy with rapid brain atrophy. Slightly more than one third of cases (18/52, 34.6%) presented with a progressive leukodystrophy, involving the corpus callosum and sparing the U fibers, variably extending to the spinal cord and cerebellar white matter, often associated with peculiar cerebral and spinal cord calcifications [18,19,20]. Less specific patterns included faint diffuse (6/52, 11.5%) or multifocal periventricular (7/52, 13.4%) white matter signal alterations. Interestingly, a third of the present patients had callosal hypoplasia, a likely underestimated feature previously described in three subjects with KARS1 variants [16, 30]. This finding is only partly related to the loss of cerebral white matter volume, as the corpus callosum in these subjects is thin but also shorter than normal. In addition, we uncovered hypoplasia of the pons in one third of subjects, and atrophy of cerebellum with prevalent vermian involvement in 20% of cases. Taken together, these neuroimaging features expand the phenotypes related to KARS1 variants, indicating a prenatal onset of the disease often associated with a rapid neurodegenerative course [31, 32].
Although a clear genotype–phenotype correlation in KARS1-related disease is lacking, we noticed some interesting associations. The KARS1 c.379T>C (p.[Phe127Leu]) variant was present in the case from family 1 in a homozygous state, and the same variant was present in compound heterozygosity in one of the cases from the Ardissone et al. report [18]. Besides the typical developmental delay and hearing loss, both cases shared other phenotypic similarity including seizures, psychomotor regression, hypotonia, spastic tetraparesis, and brain calcifications. The KARS1 c.683C>T (p.[Pro228Leu]) variant was present in compound heterozygosity in family 9 and a report by Scheidecker et al. [21], and both families manifested ataxia, visual impairment, and dystonia. Identifying and characterizing homozygous individuals with these variants in the future would be of interest to further evaluate this potential association.
Biallelic KARS1 variants have emerged as a cause of rare early-onset childhood neurodegenerative disorders presenting with a multiorgan dysfunction suggestive of mitochondrial disorders but usually with normal biochemical assays. Therefore, in addition to the initial association of biallelic KARS1 variants with Charcot–Marie–Tooth disease and nonsyndromic hearing loss in single isolated cases (2/50), an early-onset complex neurological and syndromic phenotype should be assigned to KARS1 in OMIM. This might yield more families with KARS1-related disease and improve understanding of its neurobiology and phenotypic spectrum.
The physiological relevance of several ARS genes has been assessed in vivo using zebrafish [33,34,35]. Here, we showed kars1 homozygous (kars1−/−) zebrafish larvae exhibit the phenotypic hallmarks associated with pathogenic variants reported in patients. In agreement with our study, Kars1 knockdown in Xenopus caused developmental defects of head and eyes; loss of myelination and reduced expression of myelin binding protein, leading to incomplete formation of white matter; and reduced brain volume suggesting an important role of KARS1 across the species [20].
Loss of ARS enzymes have been shown to activate the p53 signaling pathway [36]. Likewise, in zebrafish, overexpression of an editing-defective valyl-tRNA synthetase (VARS1) activates the p53 signaling pathway, and increased expression of targets such as cyclin-dependent kinase inhibitor p21 (cdkn1a) and gadd45 leading to cell death [37]. Kars1−/− mutants show upregulation of p53 signaling and apoptosis pathway genes. Interestingly, upregulation of p53 signaling has been connected to apoptosis of human oligodendrocytes (a type of myelin-forming cells) [38], and cuprizone- [39] or compressed spinal cord injury- [40] induced demyelination (one of the categories in leukodystrophy classification) in mice. Genetic or pharmacological inhibition of p53 decreased the susceptibility of cuprizone-induced demyelination and increased the survival rate of oligodendrocytes [39]. These data and our current findings emphasize that p53 pathway inhibitors might represent potential therapeutics for KARS1 patients.
One of the limitations of this study is lack of functional variant analysis in zebrafish. However, our structural modeling data shows 27/36 variants occur in the aminoacylation domain, and therefore, likely affect enzyme activity. Since KARS1 is a multifunctional protein, variants in this study could affect the canonical as well as noncanonical functions of the protein.
In summary, our findings underscore a conserved and unique requirement for ARS during nervous system and muscle development to prevent p53-mediated apoptosis and disease. Our disease model offers promising avenues to explore the biallelic contributions of KARS1, and similar approaches could be exploited to understand a number of diseases associated variants in essential genes.
Data availability
Human variant data included in this study have been deposited in Leiden Open Variation Database (LOVD) and available through following accession numbers: 0000685774, 0000685798, 0000685800, 0000685801, 0000685802, 0000685803, 0000685804, 0000685805. Reagents related to zebrafish experiments described in this paper are available upon request.
References
Meyer-Schuman R, Antonellis A. Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet. 2017;26(R2):R114–27.
McLaughlin HM, Sakaguchi R, Liu C, Igarashi T, Pehlivan D, Chu K, et al. Compound heterozygosity for loss-of-function lysyl-tRNA synthetase mutations in a patient with peripheral neuropathy. Am J Hum Genet. 2010;87:560–6.
Scheper GC, van der Klok T, van Andel RJ, van Berkel CG, Sissler M, Smet J, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007;39:534–9.
Messmer M, Florentz C, Schwenzer H, Scheper GC, van der Knaap MS, Maréchal-Drouard L, et al. A human pathology-related mutation prevents import of an aminoacyl-tRNA synthetase into mitochondria. Biochem J. 2011;433:441–6.
Maffezzini C, Laine I, Dallabona C, Clemente P, Calvo-Garrido J, Wibom R, et al. Mutations in the mitochondrial tryptophanyl-tRNA synthetase cause growth retardation and progressive leukoencephalopathy. Mol Genet Genomic Med. 2019;7:e654.
Götz A, Tyynismaa H, Euro L, Ellonen P, Hyötyläinen T, Ojala T, et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am J Hum Genet. 2011;88:635–42.
Almalki A, Alston CL, Parker A, Simonic I, Mehta SG, He L, et al. Mutation of the human mitochondrial phenylalanine-tRNA synthetase causes infantile-onset epilepsy and cytochrome c oxidase deficiency. Biochim Biophys Acta. 2014;1842:56–64.
Latour P, Thauvin-Robinet C, Baudelet-Méry C, Soichot P, Cusin V, Faivre L, et al. A major determinant for binding and aminoacylation of tRNA(Ala) in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 2010;86:77–82.
McLaughlin, HM., Sakaguchi, R., Giblin, W., Wilson, TE., Biesecker, L. & NISC Comparative Sequencing Program et al. A recurrent loss-of-function alanyl-tRNA synthetase (AARS) mutation in patients with Charcot-Marie-Tooth disease type 2N (CMT2N). Hum Mutat 33, 244–53 (2012).
Gonzalez M, McLaughlin H, Houlden H, Guo M, Yo-Tsen L, Hadjivassilious M, et al. Exome sequencing identifies a significant variant in methionyl-tRNA synthetase (MARS) in a family with late-onset CMT2. J Neurol Neurosurg Psychiatry. 2013;84:1247–9.
Safka Brozkova D, Deconinck T, Griffin LB, Ferbert A, Haberlova J, Mazanec R, et al. Loss of function mutations in HARS cause a spectrum of inherited peripheral neuropathies. Brain 2015;138(Pt 8):2161–72.
Oprescu SN, Griffin LB, Beg AA, Antonellis A. Predicting the pathogenicity of aminoacyl-tRNA synthetase mutations. Methods 2017;113:139–51.
Tolkunova E, Park H, Xia J, King MP, Davidson E. The human lysyl-tRNA synthetase gene encodes both the cytoplasmic and mitochondrial enzymes by means of an unusual alternative splicing of the primary transcript. J Biol Chem. 2000;275:35063–9.
Santos-Cortez RL, Lee K, Azeem Z, Antonellis PJ, Pollock LM, Khan S, et al. Mutations in KARS, encoding lysyl-tRNA synthetase, cause autosomal-recessive nonsyndromic hearing impairment DFNB89. Am J Hum Genet. 2013;93:132–40.
Zhou XL, He LX, Yu LJ, Wang Y, Wang XJ, Wang ED, et al. Mutations in KARS cause early-onset hearing loss and leukoencephalopathy: Potential pathogenic mechanism. Hum Mutat. 2017;38:1740–50.
McMillan HJ, Humphreys P, Smith A, Schwartzentruber J, Chakraborty P, Bulman DE, et al. Congenital visual impairment and progressive microcephaly due to Lysyl-transfer ribonucleic acid (RNA) synthetase (KARS) mutations: The expanding phenotype of aminoacyl-transfer RNA synthetase mutations in human disease. J Child Neurol. 2015;30:1037–43.
Verrigni D, Diodato D, Di Nottia M, Torraco A, Bellacchio E, Rizza T, et al. Novel mutations in KARS cause hypertrophic cardiomyopathy and combined mitochondrial respiratory chain defect. Clin Genet. 2017;91:918–23.
Ardissone A, Tonduti D, Legati A, Lamantea E, Barone R, Dorboz I, et al. KARS-related diseases: progressive leukoencephalopathy with brainstem and spinal cord calcifications as new phenotype and a review of literature. Orphanet J Rare Dis. 2018;13:45.
Sun C, Song J, Jiang Y, Zhao C, Lu J, Li Y, et al. Loss-of-function mutations in Lysyl-tRNA synthetase cause various leukoencephalopathy phenotypes. Neurol Genet. 2019;5:e565.
Itoh M, Dai H, Horike SI, Gonzalez J, Kitami Y, Meguro-Horike M, et al. Biallelic KARS pathogenic variants cause an early-onset progressive leukodystrophy. Brain 2019;142:560–73.
Scheidecker, S., Bär, S., Stoetzel, C., Geoffroy, V., Lannes, B. & Rinaldi, B. et al. Mutations in KARS cause a severe neurological and neurosensory disease with optic neuropathy. Hum Mutat 2019;40:1826–40.
Dickinson ME, Flenniken AM, Ji X, Teboul L, Wong MD, White JK, et al. High-throughput discovery of novel developmental phenotypes. Nature 2016;537:508–14.
Murray CR, Abel SN, McClure MB, Foster J, Walke MI, Jayakar P, et al. Novel causative variants in DYRK1A, KARS, and KAT6A associated with intellectual disability and additional phenotypic features. J Pediatr Genet. 2017;6:77–83.
Lieber DS, Calvo SE, Shanahan K, Slate NG, Liu S, Hershman SG, et al. Targeted exome sequencing of suspected mitochondrial disorders. Neurology 2013;80:1762–70.
Fay RR, Popper AN. Evolution of hearing in vertebrates: the inner ears and processing. Hear Res. 2000;149:1–10.
Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci USA. 2008;105:11043–9.
Hyeon DY, Kim JH, Ahn TJ, Cho Y, Hwang D, Kim S. Evolution of the multi-tRNA synthetase complex and its role in cancer. J Biol Chem. 2019;294:5340–51.
Kohda M, Tokuzawa Y, Kishita Y, Nyuzuki H, Moriyama Y, Mizuno Y, et al. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12:e1005679.
Fuchs SA, Schene IF, Kok G, Jansen JM, Nikkels P, van Gassen K, et al. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet Med. 2019;21:319–30.
Vargas A, Rojas J, Aivasovsky I, Vergara S, Castellanos M, Prieto C, et al. Progressive early-onset leukodystrophy related to biallelic variants in the KARS gene: The first case described in Latin America. Genes 2020;11:1437.
Edvardson S, Shaag A, Kolesnikova O, Gomori JM, Tarassov I, Einbinder T, et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am J Hum Genet. 2007;81:857–62.
Mendes MI, Green LMC, Bertini E, Tonduti D, Aiello C, Smith D, et al. RARS1-related hypomyelinating leukodystrophy: Expanding the spectrum. Ann Clin Transl Neurol. 2020;7:83–93.
Malissovas N, Griffin LB, Antonellis A, Beis D. Dimerization is required for GARS-mediated neurotoxicity in dominant CMT disease. Hum Mol Genet. 2016;25:1528–42.
Siekierska A, Stamberger H, Deconinck T, Oprescu SN, Partoens M, Zhang Y, et al. Biallelic VARS variants cause developmental encephalopathy with microcephaly that is recapitulated in vars knockout zebrafish. Nat Commun. 2019;10:708.
Friedman J, Smith DE, Issa MY, Stanley V, Wang R, Mendes MI, et al. Biallelic mutations in valyl-tRNA synthetase gene VARS are associated with a progressive neurodevelopmental epileptic encephalopathy. Nat Commun. 2019;10:707.
Fukushima K, Motomura S, Kuraoka A, Nakano H, Nishimoto T. A single point mutation of hamster aminoacyl-tRNA synthetase causes apoptosis by deprivation of cognate amino acid residue. Genes Cells. 1996;1:1087–99.
Song Y, Shi Y, Carland TM, Lian S, Sasaki T, Schork NJ, et al. p53-Dependent DNA damage response sensitive to editing-defective tRNA synthetase in zebrafish. Proc Natl Acad Sci USA. 2016;113:8460–5.
Ladiwala U, Li H, Antel JP, Nalbantoglu J. p53 Induction by tumor necrosis factor-alpha and involvement of p53 in cell death of human oligodendrocytes. J Neurochem. 1999;73:605–11.
Li J, Ghiani CA, Kim JY, Liu A, Sandoval J, DeVellis J, et al. Inhibition of p53 transcriptional activity: a potential target for future development of therapeutic strategies for primary demyelination. J Neurosci. 2008;28:6118–27.
Ma L, Yu HJ, Gan SW, Gong R, Mou KJ, Xue J, et al. p53-Mediated oligodendrocyte apoptosis initiates demyelination after compressed spinal cord injury by enhancing ER-mitochondria interaction and E2F1 expression. Neurosci Lett. 2017;644:55–61.
Acknowledgements
This research is supported by a grant from National Institutes of Health (NIH) GM103636 (Project 3), and the Presbyterian Health Foundation (PHF) (G.K.V.); NIH grants R01NS048453, R01NS052455 (J.G.G.); Intramural Funding (fortüne) at the University of Tübingen (2545-1-0 to B.V.) and the Ministry of Science, Research and Art Baden-Württemberg (to B.V.). Sequencing and analysis were provided by the Broad Institute (UM1 HG008900, R01HG009141) and Yale Center for Mendelian Disorders (U54HG006504 to M. Gunel). This research was made possible in part through the 100,000 Genomes Project, UK.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: S.J.L., B.V., R.M., M.S.Z., V.S., F.A., J.G.G., G.K.V. Data curation: B.V., R.K., R.M., M.S., P.B., A.A., A.T.P., G.A., J.I.E.V., H.A.D.H., N.M., A.O., J.T., D.L.R., J.M., T.S., A.R., M.S.Z., G.K.V. Formal analysis: S.J.L., B.V., P.G.B., R.K., C.P., M.S., V.S., P.V., P.B., F.A.l., A.T.P., N.M., J.M., L.S.P., D.V.R., E.T., H.Z.E., S.M., T.B.P., H.Y., J.D.W., M.B., B.F., S.K.N. Funding acquisition: B.V., J.G.G., G.K.V. Investigation: A.O., J.T., D.L.R., T.S., B.T., H.G., F.A.l., F.A.S., A.S., K.S., M.G.H., M.K., J.L.M., H.H., E.G.K., F.S.A., T.H., M.S.Z., J.G.G. Project administration: G.K.V. Resources: G.E.R.C. Supervision: B.V., M.G.H., M.K., H.H., F.S.A., T.H., J.G.G., G.K.V. Validation: B.V., R.K., R.M., M.S., P.B., G.A., J.I.E.V., H.A.D.H., N.M., A.O., J.T., D.V.R., A.R., E.T., M.S.Z. Visualization S.D., M.J. Writing—original draft: S.J.L., G.V., B.V., M.S.Z., P.G.B., V.S., M.J., and M.S., Writing—review & editing: S.M.B.
Corresponding author
Ethics declarations
Ethics Declaration
Informed written consent including use of pictures and videos was obtained prior to study inclusion. This study was performed under the tenets of the Declaration of Helsinki and approved by the Ethics Commission of the University of Wuerzburg (46/15), University of Tübingen (197/2019BO01), and University of California–San Diego (140028). All experimental animal care was performed in accordance with institutional and NIH guidelines and regulations. The study protocol was approved by the Institutional Animal Care and Use Committee of Oklahoma Medical Research Foundation, protocol 17-02.
Disclaimer
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USUHS); The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.; the Department of Defense (DoD); the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Competing interests
H.Z.E., T.B.P., E.T., S.M., and H.Y. are employees of GeneDx, Inc. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, SJ., Vona, B., Barbalho, P.G. et al. Biallelic variants in KARS1 are associated with neurodevelopmental disorders and hearing loss recapitulated by the knockout zebrafish. Genet Med 23, 1933–1943 (2021). https://doi.org/10.1038/s41436-021-01239-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-021-01239-1
This article is cited by
-
Evaluating the association of biallelic OGDHL variants with significant phenotypic heterogeneity
Genome Medicine (2023)
-
The tRNA regulome in neurodevelopmental and neuropsychiatric disease
Molecular Psychiatry (2022)
-
Specialist multidisciplinary input maximises rare disease diagnoses from whole genome sequencing
Nature Communications (2022)