Abstract
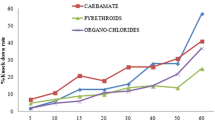
Regular monitoring of insecticide resistance among vector mosquitoes and their mechanism are very important for formulation of effective vector control measures. Insecticide resistance developed due to metabolic resistance which is mediated by the enhanced production of different detoxifying enzymes resulting in sequestration or biodegradation of insecticides. Present study was designed to assess any evidence of metabolic resistance among four major JE vectors - Cx. tritaeniorhynchus, Cx. vishnui, Cx. pseudovishnui, and Cx. gelidus from northern districts of West Bengal, India. A total of 92 adults per species of JE vectors in each study districts were analysed to measure the activity/levels of acetylcholinesterase (AChE), non-specific esterase (ESTs), glutathione-S-transferases (GSTs), and cytochrome P450s mono-oxygenase (CytP450s) according to WHO protocol. A significant increase in mean AChE activity without propoxur were observed among most of the JE vector species. All four species showed significantly elevated AChE activity with propoxur, except Cx. gelidus of Darjeeling and Uttar Dinajpur. More than 70% of Cx. tritaeniorhynchus showed homozygous resistant (RR) genotype to propoxur-inhibited residual activity of AChE but Cx. gelidus, Cx. pseudovishnui and Cx. vishnui showed either homozygous sensitive (SS) or heterozygous resistant (RS) genotype. More than 50% and 80% population of all species showed an elevated level of both alpha, beta ESTs and GSTs. CytP450s activity was also higher among all species. The present study suggests that the development of resistance against different classes of insecticides among four major JE vector species is becoming a matter of concern that should be monitored closely.





Similar content being viewed by others
Availability of data and material
All study data and material are available within the manuscript.
References
Anuradha SK, Surekha YA, Sathyanarayan MS, Suresh S, Satish P, Mariraj J, Krishna S, Rf R (2011) Epidemiological aspects of Japanese encephalitis in Bellary, Karnataka. India Int J Biologic Med Res 2(3):691–695
Bhattacharyya DR, Handique R, Prakash A, Dutta P, Mahantam J, Srivastava VK (1996) Insecticide susceptibility status of potential vectors of Japanese encephalitis in Dibrugarh district, Assam. J Commun Dis 28:67–69
Bourguet D, Capela R, Raymond M (1996) An insensitive acetylcholinesterase in Culex pipiens (Diptera:Culicidae) from Portugal. J Econ Entomol 89:1060–1066
Brewer KK, Keil CB (1989) A mixed function oxidase factor contributing to permethrin and dichlorvos resistance in Lycoriella mali (Fitch) (Diptera: Sciaridae). Pesticide Sci 26:29–39
Brogdon WG, Barber AM (1990) Microplate assay of glutathione s-transferase activity for resistance detection in single-mosquito triturates. Comp Biochem Physiol Part b: Comp Biochem 96(2):339–342
Brogdon WG (1989) Biochemical resistance detection: an alternative to bioassay. Parasitol Today 5:56–60
Brooke BD, Kloke G, Hunt RH, Koekemoer LL, Temu EA, Taylor ME, Small G, Hemingway J, Coetzee M (2001) Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bull World Health Organization 91:265–272
Casida JE, Durkin KA (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annual Rev Entomol 58:99–117
Che-Mendoza A, Penilla RP, Rodrı´guez DA, (2009) Insecticide resistance and glutathione S-transferases in mosquitoes: a review. African J Biotechnol 8:1386–1397
Fonseca-Gonza´lez I, Quin˜ones ML, McAllister J, Brogdon WG, (2009) Mixed function oxidases and esterases associated with cross-resistance between DDT and lambda-cyhalothrin in Anopheles darlingi Root 1926 populations from Colombia. Memórias Instituto Oswaldo Cruz 104:18–26
Hemingway J, Karunaratne SH (1998) Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med Vet Entomol 12(1):39–45
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annual Rev Entomol 45:371–391
Hemingway J (1998) Techniques to detect insecticide resistance mechanisms (field and laboratory manual). WHO/CDS/CPC/MAL/98.6. World Health Organization, Geneva
Hemingway J, Malcolm CA, Kisson KE, Boddington RG, Curtis CF, Hill N (1985) The biochemistry of insecticide resistance in Anopheles sacharovi: Comparative studies with a range of insecticide susceptible and resistance Anopheles and Culex species. Pesticide Biochem Physiol 24:68–76
Hemingway J, Miyamoto J, Herath PRJ (1991) A possible novel link between organophosphorus and DDT insecticide resistance genes in Anopheles: supporting evidence from fenitrothion metabolism studies. Pesticide Biochem Physiol 39:49–56
Kanojia PC, Shetty PS, Geevargheese G (2003) A long term study on vector abundance of Japanese encephalitis in Gorakhpur district, Uttar Pradesh. Ind J Med Res 117:104–110
Karunaratne SHPP, Hemingway J (2000) Insecticide resistance spectra and resistance mechanisms in populations of Japanese encephalitis vector mosquitoes, Culex tritaeniorhynchus and Cx. gelidus, in Sri Lanka. Med Vet Entomol 14:430–436
Karunaratne SHPP, Hemingway J (2001) Malathion resistance and prevalence of malathion carboxylesterase mechanism in populations of mosquito vectors of disease in Sri Lanka. Bull World Health Orga 79:1060–1064
Karunaratne SHPP, Vaughan A, Paton MG, Hemingway J (1998) Amplification of a serine esterase gene is involved in insecticide resistance in Sri Lankan Culex tritaeniorhynchus. Insect Mol Biol 7:307–315
Khan SA, Dutta P, Narainm K, Srivastava VK (1997) Studies on day-time resting habits of JE Vector mosquitoes in upper Assam with a note on insecticide susceptibility status. J Commun Dis 29:367–370
Koou SY, Chong CS, Vythilingam I, Lee CY, Lee-Ching NG (2014) Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasit Vectors 7:471
Kulkarni SM, Geevarghese G, Geoge PJ (1992) Susceptibility status of five species of Japanese encephalitis vectors to insecticides from Kolar district, Karnataka. Ind J Med Res 95:297–300
Liu N (2015) Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annual Rev Entomol 60:537–559
Low VL, Chen CD, Lee HL, Tan TK, Chen CF, Leong CS, Lim YAL, Lim PE, Norma-Rashid Y, Sofian-Azirun M (2013) Enzymatic Characterization of Insecticide Resistance Mechanisms in Field Populations of Malaysian Culex quinquefasciatus Say (Diptera: Culicidae). PLoS ONE 8(11): e79928
Misra BR, Gore M (2015) Malathion Resistance Status and Mutations in Acetylcholinesterase Gene (Ace) in Japanese Encephalitis and Filariasis Vectors from Endemic Area in India. J Med Entomol 52(3):442–446
National Vector Borne Disease Control Programme (2016) Compendium on Entomological Surveillance & Vector Control in India. Delhi. https://nvbdcp.gov.in/WriteReadData/l892s/Compendium-Entomological-Surveillance&Vector-Control-India.pdf
National Vector Borne Disease Control Programme (2014) Operational Guideline for National Programme for Prevention and Control of Japanese Encephalitis/Acute Encephalitis Syndrome. https://nvbdcp.gov.in/WriteReadData/l892s/JE-AES-Prevention-Control(NPPCJA).pdf
Pathirage DRK, Karunaratne SHPP, Senanayake SC, Karunaweera ND (2020) Insecticide susceptibility of the sand fly leishmaniasis vector Phlebotomus argentipes in Sri Lanka. Parasit Vectors 13:246. https://doi.org/10.1186/s13071-020-04117-y
Peiris HTR, Hemingway J (1993) Characterization and inheritance of elevated esterases in organophosphorus and carbamate insecticide resistant Culex quinquefasciatus (Diptera: Culicidae) from Sri Lanka. Bull Entomol Res 83:127–132
Perera MDB, Hemingway J, Karunaratne SHPP (2008) Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malar J 7:168. https://doi.org/10.1186/1475-2875-7-168
Rai P, Bharati M, Subba A, Saha D (2019) Insecticide resistance mapping in the vector of lymphatic filariasis, Culex quinquefasciatus Say from northern region of West Bengal. India Plos ONE 14(5):e0217706. https://doi.org/10.1371/journal.pone.0217706
Ranson H, Hemingway J (2005) Mosquito glutathione transferases. Methods Enzymol 401:226–241
Ranson H, Rossiter L, Ortelli F, Jensen B, Wang X, Roth CW, Collins FH, Hemingway J (2001) Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem J 359:295–304
Reuben R, Tewari SC, Hiriyan J, Akiyama J (1994) Illustrated keys to species of Culex (Culex) associated with Japanese Encephalitis on Southeast Asia (Diptera: Culicidae). Mos System 26(2):75–96
Rice CM (1996) Flaviviridae: The viruses and their replication. Fields Virol 3:931–959
Rivi M, Monti V, Mazzoni E, Cassanelli S, Panini M, Anaclerio M, Cigolini M, Corradetti B, Bizzaro D, Mandrioli M, Manicardi GC (2013) A1–3 chromosomal translocations in Italian populations of the peach potato aphid Myzus persicae (Sulzer) not linked to esterase-based insecticide resistance. Bull Entomol Res 103:278–285
Saha P, Ballav S, Chatterjee M, Ganguly S, Sarker M, Biswas AK, Pramanik T, Basu N, Maji AK (2018) The Status of Susceptibility of Japanese Encephalitis Vectors to Insecticides in Endemic Areas of Northern Districts of West Bengal, India. Japan J Infect Dis 71:91–98
Sarkar M, Bhattacharyya IK, Borkotoki A, Goswami D, Rabha B, Baruah I, Srivastava RB (2009) Insecticide resistance and detoxifying enzyme activity in the principal bancroftian filariasis vector, Culex quinquefasciatus in north-eastern India. Med Vet Entomol 23:122–131
Somwang P, Yanola J, Suwan W, Walton C, Lumjuan N, Prapanthadara LA, Somboon P (2011) Enzymes based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol Res 109:531–537
Surendran SN, Karunaratne SHPP, Adamsn Z, Hemingway J, Hawkes NJ (2005) Molecular and biochemical 399 characterization of a sand fly population from Sri Lanka: evidence for insecticide resistance due to altered esterases and insensitive acetylcholinesterase. Bull Entomol Res 95:371–380
Tyagi BK, Munirathinam A, Krishnamoorthi R, Venkatesh A (2012) A field based hand book of identification keys to mosquitoes of public health importance in India. Centre for research in Medical Entomology Madurai, India, pp 8–23
Vontas JG, Small GJ, Hemingway J (2001) Glutathione S-transferases as antioxidant 436 defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J 357:65–72
Word Health Organization (2006) Guidelines for Prevention and Control of Japanese Encephalitis. WHO, Genva
World Health Organization (1980) Resistance of vectors of disease to 439 pesticides.5th report of expert committee on vector biology and control. WHO Tech Rep Ser 655 Geneva Switzerland. https://apps.who.int/iris/handle/10665/37432
World Health Organization (1998) Techniques to detect insecticide resistance mechanisms. WHO, Geneva. https://apps.who.int/iris/handle/10665/83780
World Health Organization (2019) WHO-Japanese Encephalitis Facts sheet. https://www.who.int/news-room/fact-sheets/details/japanese-encephalitis
Zayed ABB, Szumlas DE, Hanafi HA, Fryauff DJ, Mostafa AA, Allam KM, Brogdon WG (2006) Use of bioassay and microplate assay to detect and measure insecticide resistance in field populations of Culex pipiens from filariasis endemic areas of Egypt. J Am Mos Control Assoc 22:473–482
Acknowledgements
We are thankful to the population of study villages for their help and support during the entomological collection.
Funding
The work was financially supported by the University Grant Commission (UGC), Government of India (NET JRF Fellowship 2018–2019, Ref. ID JUNE18-343757).
Author information
Authors and Affiliations
Contributions
AKM, PS and SKG designed the study; SB, AAS, PS collected the entomological samples; SB, AAS, MC, PS, AKM performed the enzyme assay, SB, AAC, MC, PS, UG, AKM perform the data analysis and interpretation; AKM, SKG, PS, MC, UG, SB prepared the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Institutional Ethics Committee of Calcutta School of Tropical Medicine, Kolkata.
Consent for publication
All authors approved the content of the manuscript.
Conflict of interest
We have no conflicts of interest concerning the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ballav, S., Chatterjee, M., Sardar, A.A. et al. Biochemical characterization of insecticide resistance in field population of major JE vectors from northern districts of West Bengal, India. Int J Trop Insect Sci 42, 661–675 (2022). https://doi.org/10.1007/s42690-021-00588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00588-3