Abstract
Previous studies have indicated that capsaicin-rich diet and cold weather have shown strong association with tumor incidence. Thus, we investigated the effects of capsaicin and cold exposure in 1,2-dimethylhydrazine (DMH)-induced colorectal cancer as well as the mechanisms underlying capsaicin and cold-induced CRC. Rats were randomly divided into four groups and received cold still water and capsaicin via intragastric gavage until the end of the experiment. The rat’s body weight, thymus weight, and food intakes were assessed. Global levels of histone H3K9, H3K18, H3K27, and H4K16 acetylation and histone deacetylase (HDACs) in colon mucosa were assessed by western blot. Expression levels of Toll-like receptors 2 (TLR2) and Toll-like receptors 4 (TLR4) were measured by western blot and reverse-transcriptase quantitative polymerase chain reaction (qPCR). We found that cold and low-dose capsaicin increased tumor numbers and multiplicity, although there were no differences in tumor incidence. Additionally, rat exposure to cold water and capsaicin display further higher levels of histone H3 lysine 9 (H3K9AC), histone H3 lysine 18 (H3K18AC), histone H3 lysine 27 (H3K27AC), and HDACs compared with the DMH and normal rats. In contrast, a considerable decrease of histone H4 lysine 16 (H4K16AC) was detected in the colon mucosa. Cold and low-dose capsaicin exposure groups were also increased TLR2 and TLR4 protein levels and mRNA levels. These results suggest that chronic cold exposure and capsaicin at a low-dose intervention exacerbate ectopic expression of global histone acetylation and TLR level, which are crucial mechanisms responsible for the progression of colorectal cancer in rats.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) has been recognized as a multi-factorial malignant disorder and remains its status as the most common cause of cancer-related death in men and women (Siegel et al. 2019). Colon cancer is caused by complex interactions of the immune system with non-modifiable (e.g., genetic and epigenetic alterations) and modifiable (e.g., environmental, diet and lifestyle) risk factors (Grazioso et al. 2019; Lao & Grady, 2011). The fact that known high penetrance gene and epigenetic alterations that are related to colorectal cancer risk explain fewer than 5% of the observed case suggested that environmental factors including diet, alcohol consumption, and ambient environment play a pivotal role in risk (Lichtenstein et al. 2000). Moreover, it is important to note that these modifications of known factors are most associated with cancer death in China (Chen et al. 2019).
Identifying the epigenetic mechanisms and their interaction with diet and Toll-like receptors is crucial to reveal the regulation of molecular pathways leading to colorectal carcinogenesis. Colorectal cancer (CRC) is characterized by the accumulation of genetic and epigenetic alterations (Okugawa et al. 2015). Epigenetic modifications have been implicated in the regulation of gene expression, without affecting the DNA sequence (Strahl and A. C. 2000). Among these modifications, histone acetylation on lysine residues is the most common histone modification. The expression level of histone acetylation is important regarding chromatin states and gene expression. Overall, lysine acetylation generally opens the chromatin and activates gene expression, whereas deacetylated is related to transcriptional repression (Berger 2007). It has previously been reported that aberrant histone acetylation was implicated in the initiation and progression of various solid tumors including CRC (Kaypee et al. 2016). Dynamic levels of reversible acetylation are mediated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). They are classified into four classes based on cellular localization and functional regulation (Annemieke et al. 2003). More and more research indicated that germline mutations of HDACs increase the risk of breast and lung cancers, and abnormal HDAC overexpression has also been observed in a wide range of malignancies, thereby serving as therapeutic targets for CRC (Glozak and Seto 2007; Singh et al. 2018). Previous study indicated that animal housing at sub-thermoneutral (22–26°C) has a profound effect on obesity, inflammation, cancer, and other metabolic disorders (Ganeshan and Chawla 2017; Hylander and Repasky 2016). For example, studies in broilers revealed that long-term cold stimulation aggravated oxidative stress, ultrastructural cardiac damage, immune dysregulation, and inflammation (Wei et al. 2018). It was also found that the cold environment substantially potentiates the development of malignancy resulting from subthermoneutral laboratory housing temperature cause mild chronic cold stress, which triggers suppression of the antitumor immune response (Kokolusa et al. 2013). Some published reports have described an anti-cancer role for capsaicin, while others have argued that capsaicin is implied as a promoting factor by inducing the growth and progression of preexisting neoplasms (Bode and Dong 2011). In vitro and in vivo experimental of CRC indicated that capsaicin exerts anti-tumor activity through induces cytotoxicity and apoptosis (Lu et al. 2010). On the other hand, Yang et al. reported that human colon carcinoma promotion involved in the Akt/mTOR/STAT-3 signaling pathway (Yang et al. 2013). Toll-like receptors (TLRs) have an important role in the activation of both innate and adaptive immunity, and aberrant activation of the TLR signal can result in disturbance of intestinal immune homeostasis, chronic inflammatory, and therefore an important modulator in the pathogenesis of CRC (Lavelle et al. 2009). It has been also demonstrated that TLR agonists have immune regulatory applications as vaccine adjuvants in cancer therapy (Moradi-Marjaneh et al. 2018). Furthermore, epigenetics has recently been proven to be a new regulator of TLR expression (Xie et al. 2018). However, much less known regarding the mechanism underlying the putative effect of potential chronic cold and capsaicin exposure contribution on the promotion and progression stages of DMH-induced colon carcinoma in rat models.
Therefore, the purpose of this research was to evaluate the expression of aberrant histone modification via the modulation of HDACs as well as the TLR signaling pathway on colon carcinogenesis.
Material and method
Chemicals and preparation
1,2-Dimethylhydrazine (DMH) was purchased from Sigma-Aldrich (St. Louis, MO, USA). DMH was weighed and dissolved in 1Mm EDTA-saline to ensure the stability of the chemical (Gungor et al. 2018). And the pH was adjusted to 7.0 each time with 1 M NaOH solution. 95% purity of capsaicin provided by Xian Ruilin Biotechnology Co (Xian, China).
Animals and treatment
Six-week-old male Wistar rats weighed between 200 and 250g were kept in SPF laboratory animal room generated from Experimental Animal Center in Guangzhou University of Chinese Medicine. The animals were given ad libitum access to water and food and housed in plastic cages in a temperature-controlled (24°C) room with a 12-h light-dark cycle. All the experimental protocols were permitted by the Institutional Animal Ethics Committee of the Guangzhou University of Chinese Medicine (No. 20130001). Based on the previously described protocol [23, 24], we made some minor revisions to the established colon carcinoma model induced by 1, 2-dimethylhydrazine (DMH) in rats. Briefly, after a period as long as 3days for adapting to the environment, rats were randomly divided into 4 groups with 10 animals each (normal control group, DMH group, capsaicin exposure group, and cold exposure group). The capsaicin exposure group was given capsaicin at 10mg/kg b.wt. every day. The cold exposure group was treated with cold distilled water (0°C) at 10mg/kg b.wt. until the end of the experiment.
Group A (normal control): Rats received basal diet, drink water, and gavage with the equivalent volume of saline once a day.
Group B (DMH): Rat was administered and subcutaneously injected with DMH (25mg/kg b.wt.) once a week for 12 weeks and also received saline once a day.
Group C (capsaicin combined with DMH): Rats were administered with DMH as in GP B and also given capsaicin (10mg/kg b.wt.) every day throughout the experiment. DMH was weighed and dissolved in normal saline to ensure the stability of the chemical before use with a final concentration of 2 %. And the pH was adjusted to 7.0 each time with 1 M NaOH solution. The preparation of DMH in other groups was the same.
Group D (DMH + cold distilled water): Rats were administered with DMH as in GP B and also given cold distilled water (10mg/kg b.wt.) via intragastric gavage continued till the end of different phases of experiments.
The appearance change of rats was monitored daily. Bodyweight and food intake of rats were recorded weekly throughout the experiment. For food consumption measurement, the model rats were provided with 500g of food in the morning. Food weight was detected at a fixed time to determine daily food intake with the last 24 h. All the rats were anesthetized with chloral hydrate and then sacrificed 30 weeks after the end of DMH administration. The experiment lasted 30 weeks. The experimental protocol is exhibited in Fig. 1.
Morphological study of colon tissues
Colons were longitudinally dissected from the anal to the cecum and then washed it with PBS. The colons were spread out on cleaning tissue paper. The number of colon tumors was recorded for tumor incidence and the size of the tumors was measured using a caliper paper. Tumor volume was calculated using the formula: tumor volume (mm3) = length (mm) × width2 (mm2)/2 as reported by Isha Rani (Isha Rani and Agnihotri 2014).
Histopathological observation
For histology, the colon sections were placed in a 15-ml conical tube filled with a 4% paraformaldehyde solution over 24 h. Colon paraffin sections were prepared by the pathology department at the Guangzhou University of Chinese Medicine. H&E staining sections were visualized through an Olympus TH4-200 (Tokyo Japan) under ×400 magnification. Microscope was classified as tumors in accordance with specific pathological criteria described by Nolte. Colonic neoplasms were classified as adenomas or adenocarcinomas (tubular or mucinous) (Nolte et al. 2016). Tumor incidence is the percentage of rats bearing the indicated type of tumor. Incidence values are represented as a percentage.
Histone extraction
We extracted the total histones from colon tumor samples (n=3/group) using the EpiQuik total histone extraction kit (EpiGentek, Farmingdale, NY, U.S.A.). We prepared histone following the manufacturer’s protocol. Briefly, 100mg colon tissues were homogenized in a mortar with liquid nitrogen, adding lysis buffer, and then incubated on ice for 30 min. The homogenized mixture was transferred into a 2-ml vial and centrifuged at 3000g for 5 min at 4°C, and then the supernatant was removed. Balance buffer was added and incubated on ice for 30 min and centrifuged at 3000g for 5 min at 4°C, and the supernatant fraction was transferred into a new vial, adding Balance-DTT buffer immediately. Protein concentrations were quantified by Bradford protein assay kit (KeyGEN, China) and the extracts were stored at −80°C.
Western bolts
For western blotting, we fractionated 30 μg of extracted protein on a 12% SDS-PAGE gel and then transferred to a polyvinylidene fluoride (PVDF) membrane with a 22-mm pore size (Millipore, MA, USA). The membrane was blocked in 5% milk PBS-T (phosphate-buffered saline with 0.1% Tween-20) for 2 h at room temperature and probed with antibodies acetylated H3-Lys9 (1:2000), acetylated H3-Lys18 (1:2000), acetylated H3-Lys27 (1:2000), acetylated H4-Lys16 (1:4000), TLR2 (1:1000), and TLR4 (1:1000) at 4°C overnight. We used horseradish peroxide (HRP)–conjugated goat anti-rabbit as the secondary antibody and histone H3, H4, and β-actin (1:5000) were used as a loading control. All antibodies were purchased from Abcam (Cambridge, UK). Band intensity was visualized by ChemiDoc TMXRS+ (Bio-Rad, USA)
Real-time quantitative RT-PCR
Total RNA was extracted from colon tumor tissues (n=6/group) using a Trizol reagent (Takara, Japan). The concentration and quality of RNA samples were evaluated with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). Reverse transcription was carried out to obtain cDNA using the Master Mix kit (Takara, Japan) following the standard protocols. The mRNA levels of TLR2 and TLR4 in colon mucosa were assessed using a Step One Plus real-time PCR system (Thermo Fisher Scientific, CFX384TM Real-time System). β-Actin was used as housekeeping gene to normalize mRNA expression. The relative levels of gene expression were enumerated using the comparative formula 2-ΔΔCt (C. Liu et al. 2018). Detailed information of the primer sequences was as follows:
5′-AGCCATGTACGTAGCCATCC-3′/3′-ACCCTCATAGATGGGCACAG-5′ for β-actin.
5′-GCTCCTGTGAACTCCTGTCC-3′/3′-GACACTCCAAGACTGAGGGC-5′ for TLR2.
5′-CCAGAGCCGTTGGTGTATCT-3 ′/3′-GGCGATACAATTCGACCTGC-5′ for TLR4.
Statistical analysis
All the data were summarized as mean±standard deviation (SD) and analyzed by SPSS 23.0. To determine if there were differences between groups, we performed the data with a one-way analysis of variance (ANOVA). Fisher’s exact test was performed to compare the tumor incidence between the different groups. We considered differences significant at P< 0.05.
Result
General observation
As shown in Fig. 2A, before 22 weeks, weekly monitoring throughout the experimental period showed no noticeable body weight loss among groups. After 22 weeks, DMH-treated rats showed a decrease in body weight as compared to control rats. Compared with the DMH-treated group, the weight of cold exposure groups was reduced, but the statistical difference among groups was not significant. No difference in food intake was found among groups by the end of weeks 2–6. The food intakes of DMH-treated rats were decreased at the rest of the experimental period. In 28–30 weeks, the food intake was increased in the cold exposure group as compared to the DMH-treated group. Conversely, the food intake was decreased in the capsaicin group in 12–18 weeks compared with that in the DMH group. Figure 2 C and E showed that the thymus weight in the cold and capsaicin exposure groups was lower than that of the normal group, although no significant difference in the thymus weight was observed among DMH-treated, cold exposure, and capsaicin treatment groups.
Bodyweight, food intake, thymus weight, and tumor volume in normal rats, cold exposure and capsaicin-treated rats. A Effects of cold exposure and capsaicin on the bodyweight of rats. B Effects of cold exposure and capsaicin on the food intake of rats. C, E Change in thymus weight and thymic morphology. D, F Macroscopic image of the colonic tumors and tumor volumes in different treatment groups. Data were expressed as the mean ± S.D. Comparisons: # control compared with DMH, * DMH compared with cold exposure and capsaicin-treated group. # P < 0.05, ## P < 0.01, * P < 0.05, ** P < 0.01
Morphological study of colon tumor and histopathological analysis
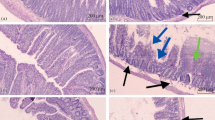
Seven months after the experiment started, no visible colon tumor was found in normal control. Most of the colonic tumors were developed in the distal colon than in the proximal colon of Wistar rats. As shown in Fig. 2 D and F, the average volume tumor of the DMH-treated group was 15.24 ± 12.45 mm3 and that of the cold exposure and capsaicin treatment groups was 34.58± 27.76 mm3 and 22.70 ± 18.02 mm3, respectively. These data suggest that there was a bigger volume in rats of the cold exposure and capsaicin treatment than the DMH treatment. There was no difference in tumor incidence among groups (Table 1). The tumor multiplicity was higher among the cold exposure and capsaicin groups as compared to the DMH group due to the increased number of tumors. A trend towards the increase of invasive tumors was observed in the cold and capsaicin group. Furthermore, the pathological type of the cold group was the most serious. Histopathological analysis showed that most tumors were either tubular adenocarcinomas or poorly differentiated mucinous adenocarcinomas (Fig. 3).
Representative sections stained with H&E showing the histopathology of the colonic neoplastic lesions. A Adenoma, characterized by non-invasive polypoid tumor projecting into the intestinal lumen, B invasive tubular adenocarcinoma, and C invasive mucinous adenocarcinoma. Both featuring invasion of abnormal tubular or mucinous structures through the muscularis mucosa or deeper layers of the intestinal wall
Effect of cold exposure and capsaicin on HDAC activity
The expression of HDAC1, 2, 3, and 8 in colonic mucosa is shown in Fig. 4 A and B. The protein expression levels of HDAC 1, 2, 3, and 8 were significantly elevated in colonic tissue from DMH-treated rats. Compared to those in the DMH group, the HDAC 1, 2, 3, and 8 expressed in the cold and capsaicin exposure groups were further increased. These findings implicated that the activation of the HDACs may be involved in the CAC induced by DMH and cold exposure and capsaicin treatment could exacerbate the expression levels of HDACs.
The effect of cold and capsaicin exposure on the protein of HDAC1-3, 8. A Representative Western immunoblot images. B Semi-quantitative analysis of HDAC1-3, 8 immunoblots was normalized by corresponding β-actin bands. Cumulative values are reported as means ± S.E. from three separate experiments. Comparisons: # control compared with DMH, *DMH compared with cold exposure and capsaicin-treated group. # P < 0.05, ## P < 0.01, * P < 0.05, ** P < 0.01
Effect of cold exposure and capsaicin on colonic tissue H3K9ac, H3K18ac, H3K27ac, and H4K16ac protein levels
The effect of cold exposure and capsaicin H3K9ac, H3K18ac, H3K27ac, and H4K16ac in colon mucosa is shown in Fig. 5. Compared to those in the control group, H3K9ac, H3K18ac, and H3K27ac in the DMH group were increased. In contrast, exposure of rats with cold water and capsaicin resulted in a loss of H4K16 acetylation in colon mucosa during colorectal carcinogenesis. Compared with the DMH group, the cold and capsaicin exposure group further increased the levels of H3K9ac and H3K27ac but had no effect on H3K18ac levels. However, the protein level of H4K16ac was lower than that in the DMH-treated group, but the statistical difference among the groups was not significant.
Western blot analysis of histone H3 and H4 modifications in the colonic mucosa of different treatment groups. A Representative Western immunoblot images are shown. B Semi-quantitative analysis of immunoblots was normalized by corresponding histone 3 or histone 4 bands. Data were presented as the mean ± S. D form three separate experiments. Comparisons: # control compared with DMH, *DMH compared with cold exposure and capsaicin-treated group. # P < 0.05, ## P < 0.01, * P < 0.05, ** P < 0.01
Effect of cold exposure and capsaicin on colonic mucosa TLR2 and TLR4 protein levels
The expression of TLR2 and TLR4 protein levels and mRNAs in colonic mucosa are shown in Fig. 6. The cold and capsaicin exposure group had significantly higher levels of TLR2 and TLR4 protein and mRNAs in colon mucosa as compared with control. Compared to those in the DMH group, expression of TLR2 and TLR4 protein levels and mRNAs in cold and capsaicin exposure groups were further increased.
Effect of cold and capsaicin exposure on the expression of TLR2 and TLR4 proteins in different treatment groups. A Representative Western immunoblot images are shown. B Semi-quantitative analysis of immunoblots was normalized by corresponding β-actin bands. C, D The mRNA expression levels of TLR2 and TLR4 in the colon tissues of rats in different groups. Data were presented as the mean ± S. D from three independent experiments. Comparisons: # control compared with DMH, *DMH compared with cold exposure and capsaicin-treated group. # P < 0.05, ## P < 0.01, * P < 0.05, ** P < 0.01
Discussion
Recent several epidemiological studies have associated cold temperatures and capsaicin consumption with an increased risk of several cancers (Du et al. 2020; Michal Freedman et al. 2015; Voskarides 2019). Our findings demonstrated that cold exposure and low-dose capsaicin administration aggravate the ectopic expression of histone acetylation level and histone-modifying enzymes through the structure of chromatin, making rats induced by DMH more susceptible to CRC. Moreover, cold and capsaicin exposure further increased TLR2 and TLR4 expression as well as increased histone H3 acetylation of TLR2 and TLR4 in the colonic mucosa of DMH-induced rats but not normal rats. These results suggest that higher HDAC expression in the colonic mucosa and ectopic expression of histone acetylation may be involved in chromatin remodeling, which might play a fundamental role in the pathogenesis of CRC.
More recently, evidence supports an emerging view that environmental factors such as diet, lifestyle, and environment are among the top risk factors that predispose to cancer. For example, males exposed to a high concentration of toxic contaminants, sperm nuclear basic proteins (SNBP), were involved in DNA oxidative damage Lettieri et al. 2020a, 2020b). DNA damage contributes to the initiation and progression of cancer by increasing cell proliferation, genetic alterations, and genomic instability (Akshay Bhat et al. 2018; Chatterjee and Walker 2017). In addition, it is also demonstrated that environmental contaminants can have transgenerational inherited effects on spermatozoa, DNA, and the molecular alterations of SNP (Lettieri et al. 2020a, 2020b). The presence of high levels of DNA damage in human spermatozoa has been correlated with infertility and childhood cancer (Bisht et al. 2017. Furthermore, it has been reported that cold environmental temperature can be potential cancer-causing factor, and people who live in the cold environment have a high risk of cancer incidence and mortality (Sharma et al. 2015). Kokolus and colleagues found that when tumor-bearing mice are housed at thermoneutral temperature (30–31°C), they observed reduced tumor formation, growth, and metastasis (Kokolusa et al. 2013). Capsaicin is the major pungent alkaloid of chili peppers (Naves et al. 2019). Conflicting epidemiological and basic research studies suggest that capsaicin exerts a role in either a protective effect or carcinogenic effect (Bode and Dong 2011; Friedman et al. 2019). Recent studies indicate the protective effect of capsaicin against various types of cancer. It has been shown that capsaicin at 50mg/kg reduced cancer risk in DMH-induced CRC rat models by inhibiting the cytotoxicity, genotoxicity, proliferation, and apoptosis of cancer cell/tissue (Caetano et al. 2018). They also showed that high-dose capsaicin decreases the proportion of tubular adenocarcinoma and tumor size in DMH-induced CRC (Caetano et al. 2021). Additionally, capsaicin suppresses the growth of numerous types of cancer cell, suggesting that it has chemopreventive activities (Li et al. 2018; Zheng et al. 2016). However, various studies have suggested a potential pro-carcinogenic effect of capsaicin use. Epidemiologic studies suggested the consumption of hot peppers, which contain variable levels of capsaicin, might increase the risk of CRC (Zhivotovskiy et al. 2012). It was reported that capsaicin has tumor-promoting activities in skin, lung, and colon cancer in different chemically induced carcinogenesis models (Geng et al. 2016; Liu et al. 2015; Nalinia et al. 1998). Another study indicated that capsaicin could significantly accelerate tumor formation and growth in 12-O-tetradecanoylphorbol-13-acetate (TPA)–induced skin carcinogenesis through the inflammation-dependent mechanism (Z. Liu et al. 2015). In vitro study showed that low concentrations of capsaicin enhanced the migratory and invasive capability of CRC cells by upregulating the expression of tumor-associated NADH oxidase (Liu et al. 2012). The results of the present study showed that chronic cold exposure and long-term application of capsaicin at 10mg/kg promote the development and progression of CRC in animal experimental models. We accordingly speculated that the anti-cancer activity of capsaicin may depend on its concentration. Further study is needed to precisely delineate the effects of capsaicin on CRC.
The overall disruption of the epigenetic landscape including alteration in DNA methylation and histone modification pattern is the most common feature of all human tumors (Nebbioso et al. 2018). DNA methylation is central to the control of retroelements in the human genome, ontogeny, and phylogeny (Yoder et al. 1997). Transcriptional regulatory sequences from retroelements, typically from LINEs, have changed with regard to methylation and activity (Schulz 2006). Many studies suggest hypomethylation of LINEs may be essential in the pathophysiology of various diseases and cancer, contributing to chromosomal instability and chromatin structure alteration (Jin et al. 2009; Vafadar-Isfahani et al. 2017). It was reported that the DNA methylation expression level of two retrotransposon sequences-LINE1 and L1 was decreased after cortical spreading depression (CDS) induction, suggesting the essential role of DNA methylation of retrotransposon in the pathological state of ischemia (Drongitis et al. 2016). By now, retroelements in diseases are implicated in a much wider range of afflictions, such as atherosclerosis and schizophrenia Kan et al. 2004; Tabaei and Tabaee 2019). Recent research has shown that regulation of specific HDAC isoforms and aberrant histone modification alterations imposed by diet might not be the only mechanisms responsible for neoplastic cell transformation but also be implicated in the development of cancer (Nebbioso et al. 2018). The overexpression of class I HDACs has been reported in many cancer types including colon carcinoma (Weichert et al. 2008). Furthermore, the expression was enhanced in strongly proliferating and poorly differentiated tumors and upregulation of HDAC correlated with poor prognosis of CRC (Ashktorab et al. 2009; Y. Li and Seto 2016). In addition, H3K9ac, H3K18ac, and H3K27ac were reported to be significantly upregulated in colorectal adenomas and cancers as compared to their normal counterparts (Karczmarski et al. 2014). Tamagawa et al. reported a decreased methylation of H3K27me2 in liver metastasis CRC in comparison with primary tumors (Tamagawa et al. 2013). Moreover, several studies have suggested that global patterns of histone modification provide additional independent information for histological subtype, prognosis, and cancer recurrence (David B. Seligson et al. 2009; Tamagawa et al. 2013). For instance, in breast cancer, low global H3K27me3 methylation levels have been described using genome-wide analyses whose expression correlated with aggressive subtypes of breast cancer (Hsieh et al. 2020). Loss cell levels of H3K4me2 and H3K9me3 demonstrated correlations with poor differentiation, shorter disease-free survival (DFS), and metastases in pancreatic cancer (Manuyakorn et al. 2010). The hypoacetylation of H3K9, H3K18, and H4K16 was strongly relevant to the clinical outcome of prostate cancer (D. B. Seligson et al. 2005). While elevated global histone acetylated histone H3 (H3ac) in colon cancer tissues was reported to predict poor overall survival in patients (Hashimoto et al. 2013), our results showed that the expression of H3K9ac, H3K18ac, and H3K27ac in the colons of DMH-induced CRC rats was significantly increased, and cold exposure and capsaicin treatment further increased this phenomenon. As compared with the DMH group, the acetylation of H4K16 was clearly downregulated in the cold exposure and capsaicin treatment groups. We postulated that upregulated HDACs lead to abnormal patterns of histone acetylation, which is associated with the deregulation of gene transcription, thereby contributing to the CRC progression. Understanding the complex biology of histone modification and its regulators is thus essential for CRC pathogenesis and treatment.
There are many molecular pathways involved in CRC development and different levels of evidence support that chronic inflammation plays an essential role in cancer development and progression (Diakos et al. 2014; Monteleone et al. 2012; Grivennikov et al. 2010). Aberrant activation of TLRs has been shown to increase the risk of colorectal cancer caused by disruption of chronic inflammation, immune response, and epithelial barrier homeostasis, which predisposes individuals to develop CRC (Cario 2010; Li et al. 2014; Xiang et al. 2012). Thus, its aberrant activation can be implicated in the pathogenesis of intestinal diseases, such as inflammatory bowel disease (IBD), colitis-associated cancer (CAC), and CRC. The abnormal activation of Toll-like receptors (TLRs) leads to an impairment of immune homeostasis, which contributes to CRC development. It is reported that TLR2 and TLR4 are upregulated in CRC and correlated with a poor prognosis in patients with CRC (Nihon-Yanagi et al. 2012; Xu et al. 2011). Additionally, it has been shown that genetic variation in TLR2 and TLR4 gene interaction with dietary factors increased susceptibility to CRC and multiple single-nucleotide polymorphisms (SNPs) in these genetic profiles, associated with CRC prognosis (Kopp et al. 2018; Slattery et al. 2012). Furthermore, recent research investigating the role of TLRs in diseases indicates that DNA methylation and histone modification epigenetic have emerged as new mechanisms involved in the regulation of TLRs (Hennessy and McKernan 2016). Takahashi et al. (Takahashi et al. 2009) suggested that epigenetic modification, including histone acetylation and DNA methylation, acts as a negative regulator of TLR4 gene transcription when associated with ZNF160, a repressive-associated transcription factor in human intestinal epithelial cells. TLR2 was reported to decrease methylation of the proximal human TLR2 promoter, resulting in an upregulation of TLR2 (Haehnel et al. 2002). Here we observed that cold and capsaicin exposure further increased the expression of TLR2 and TLR4 in the colon mucosa and this could be related to the epigenetic mechanism. Histone modification might be one of the regulators of TLR2 and TLR4. Further studies are necessary to identify the potential genetic locus regulated by histone modification upon cold exposure and administration of capsaicin in DMH-induced CRC through chromatin immunoprecipitation (ChIP) and next-generation sequencing (ChIP-seq) assay.
Conclusion
In summary, as shown in Fig. 7, the present study indicated that cold exposure and long-term administration of capsaicin at a low dose further aggravate the abnormal expression of HDACs and global histone modification as well as TLRs and that such changes are strongly correlated with the development and progression of CRC. A better understanding of the epigenetics and TLRs on cold exposure and capsaicin treatment rats may help to clarify the mechanism of tumorigenesis which could afford a novel therapeutic approach for CRC patients. Lastly, further investigations are required to understand the epigenetic mechanisms of TLR regulation and the role of TLRs in the pathogenesis, prevention, and treatment of CRC.
Schematic diagram depicting the role of histone acetylation and Toll-like receptors in promoting colorectal cancer pathogenesis. In comparison with the normal rats, rats exposed to cold and capsaicin have higher levels of HDACs activity in the colon mucosa, which results in abnormal expression of histone H3-K9, H3-K18, H3-K27, and H4-K16 acetylation. The innately higher levels are associated with the relaxation of chromatin of TLR2 and TLR4 genes. These results implicate a crucial role of histone acetylation–induced chromatin remodeling and can also positively regulate the expression of TLRs, thereby exacerbating the CRC progression
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akshay Bhat SH, Pal A, Jha S, Taneja R (2018) Stressing the (epi)genome: dealing with ROS in cancer. Antioxid Redox Signal 29(13):1273–1292. https://doi.org/10.1089/ars.2017.7158)
Annemieke JM.. De RUIJTER AHVG., Huib N. CARON, Stephan KEMP and Andre, & Kuilenburg BPV. (2003). Histone deacetylases (HDACs): characterization of the classical HDAC family. 370, 737–749
Ashktorab H, Belgrave K, Hosseinkhah F, Brim H, Nouraie M, Takkikto M, Hewitt S, Lee EL, Dashwood RH, Smoot D (2009) Global histone H4 acetylation and HDAC2 expression in colon adenoma and carcinoma. Dig Dis Sci 54(10):2109–2117. https://doi.org/10.1007/s10620-008-0601-7
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447(7143):407–412. https://doi.org/10.1038/nature05915
Bisht S, Faiq M, Tolahunase M, Dada R (2017) Oxidative stress and male infertility. Nat Rev Urol 14(8):470–485. https://doi.org/10.1038/nrurol.2017.69
Bode AM, Dong Z (2011) The two faces of capsaicin. Cancer Res 71(8):2809–2814. https://doi.org/10.1158/0008-5472.CAN-10-3756
Caetano BFR, Tablas MB, Pereira NEF, de Moura NA, Carvalho RF, Rodrigues MAM, Barbisan LF (2018) Capsaicin reduces genotoxicity, colonic cell proliferation and preneoplastic lesions induced by 1,2-dimethylhydrazine in rats. Toxicol Appl Pharmacol 338:93–102. https://doi.org/10.1016/j.taap.2017.11.008
Caetano BFR, Tablas MB, Ignoti MG, de Moura NA, Romualdo GR, Barbisan LF, Rodrigues MAM (2021) Capsaicin lacks tumor-promoting effects during colon carcinogenesis in a rat model induced by 1,2-dimethylhydrazine. Environ Sci Pollut Res Int 28(2):2457–2467. https://doi.org/10.1007/s11356-020-10683-6
Cario E (2010) Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 16(9):1583–1597. https://doi.org/10.1002/ibd.21282
Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58(5):235–263. https://doi.org/10.1002/em.22087
Chen W, Xia C, Zheng R, Zhou M, Lin C, Zeng H, Zhang S, Wang L, Yang Z, Sun K, Li H, Brown MD, Islami F, Bray F, Jemal A, He J (2019) Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health 7(2):257–269. https://doi.org/10.1016/s2214-109x(18)30488-1
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. The Lancet Oncology 15(11):e493–e503. https://doi.org/10.1016/s1470-2045(14)70263-3
Drongitis D, Rainone S, Piscopo M, Viggiano E, Viggiano A, De Luca B et al (2016) Epigenetics and cortical spreading depression: changes of DNA methylation level at retrotransposon sequences. Mol Biol Rep 43(8):755–760. https://doi.org/10.1007/s11033-016-4000-4
Du Y, Lv Y, Zha W, Hong X, Luo Q (2020) Chili consumption and risk of gastric cancer: a meta-analysis. Nutr Cancer 73:1–10. https://doi.org/10.1080/01635581.2020.1733625
Friedman JR, Richbart SD, Merritt JC, Brown KC, Denning KL, Tirona MT, Valentovic MA, Miles SL, Dasgupta P (2019) Capsaicinoids: multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed Pharmacother 118:109317. https://doi.org/10.1016/j.biopha.2019.109317
Ganeshan K, Chawla A (2017) Warming the mouse to model human diseases. Nat Rev Endocrinol 13(8):458–465. https://doi.org/10.1038/nrendo.2017.48
Geng S, Zheng Y, Meng M, Guo Z, Cao N, Ma X et al (2016) Gingerol reverses the cancer-promoting effect of capsaicin by increased TRPV1 level in a urethane-induced lung carcinogenic model. J Agric Food Chem 64(31):6203–6211. https://doi.org/10.1021/acs.jafc.6b02480
Glozak M, Seto E (2007) Histone deacetylases and cancer. Oncogene 2007(26):5420–5432. https://doi.org/10.1038/sj.onc.1210610
Grazioso TP, Brandt M, Djouder N (2019) Diet, microbiota, and colorectal cancer. iScience 21:168–187. https://doi.org/10.1016/j.isci.2019.10.011
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899. https://doi.org/10.1016/j.cell.2010.01.025
Gungor H, Ilhan N, Eroksuz H (2018) The effectiveness of cyclooxygenase-2 inhibitors and evaluation of angiogenesis in the model of experimental colorectal cancer. Biomed Pharmacother 102:221–229. https://doi.org/10.1016/j.biopha.2018.03.066
Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M (2002) Transcriptional regulation of the human toll-like receptor 2 gene in monocytes and macrophages. J Immunol 168(11):5629–5637. https://doi.org/10.4049/jimmunol.168.11.5629
Hashimoto T, Yamakawa M, Kimura S, Usuba O, Toyono M (2013) Expression of acetylated and dimethylated histone H3 in colorectal cancer. Dig Surg 30(3):249–258. https://doi.org/10.1159/000351444
Hennessy C, McKernan DP (2016) Epigenetics and innate immunity: the ‘unTolld’ story. Immunol Cell Biol 94(7):631–639. https://doi.org/10.1038/icb.2016.24
Hsieh IY, He J, Wang L, Lin B, Liang Z, Lu B, Chen W, Lu G, Li F, Lv W, Zhao W, Li J (2020) H3K27me3 loss plays a vital role in CEMIP mediated carcinogenesis and progression of breast cancer with poor prognosis. Biomed Pharmacother 123:109728. https://doi.org/10.1016/j.biopha.2019.109728
Hylander BL, Repasky EA (2016) Thermoneutrality, mice, and cancer: a heated opinion. Trends Cancer 2(4):166–175. https://doi.org/10.1016/j.trecan.2016.03.005
Isha Rani KV, Agnihotri N (2014) Supplementation of fish oil augments efficacy and attenuates toxicity of 5-fluorouracil in 1,2-dimethylhydrazine dihydrochloride/dextran sulfate sodium-induced colon carcinogenesis. Cancer Chemother Pharmacol 74(2):309–322. https://doi.org/10.1007/s00280-014-2497-6)
Jin M, Kawakami K, Fukui Y, Tsukioka S, Oda M, Watanabe G, Takechi T, Oka T, Minamoto T (2009) Different histological types of non-small cell lung cancer have distinct folate and DNA methylation levels. Cancer Sci 100(12):2325–2330. https://doi.org/10.1111/j.1349-7006.2009.01321.x
Kan PX, Popendikyte V, Kaminsky ZA, Yolken RH, Petronis A (2004) Epigenetic studies of genomic retroelements in major psychosis. Schizophr Res 67(1):95–106. https://doi.org/10.1016/j.schres.2003.09.004
Karczmarski J, Rubel T, Paziewska A, Mikula M, Bujko M, Kober P et al (2014) Histone H3 lysine 27 acetylation is altered in colon cancer. Clin Proteomics 11(24):1–10
Kaypee S, Sudarshan D, Shanmugam MK, Mukherjee D, Sethi G, Kundu TK (2016) Aberrant lysine acetylation in tumorigenesis: implications in the development of therapeutics. Pharmacol Ther 162:98–119. https://doi.org/10.1016/j.pharmthera.2016.01.011
Kokolusa KM, Capitanoa ML, Leea C-T, Enga JW-L, Waighta JD, Hylandera BL et al (2013) Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proceeding if the Nation Academy of Science of the United State of America 110(50):20176–20181
Kopp TI, Vogel U, Tjonnel A, Andersen V (2018) Meat and fiber intake and interaction with pattern recognition receptors (TLR1, TLR2, TLR4,and TLR10) in relation to colorectal cancer in a Danish prospective, case-cohort study. The American journal of clinical nutriention 107(3):465–479. https://doi.org/10.1093/ajcn/nqx011
Lao VV, Grady WM (2011) Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol 8(12):686–700. https://doi.org/10.1038/nrgastro.2011.173
Lavelle EC, Murphy C, O’Neill LAJ, Creagh EM (2009) The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol 3(1):17–28. https://doi.org/10.1038/mi.2009.124
Lettieri G, D'Agostino G, Mele E, Cardito C, Esposito R, Cimmino A et al (2020a) Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int J Mol Sci 21(12). https://doi.org/10.3390/ijms21124198
Lettieri G, Marra F, Moriello C, Prisco M, Notari T, Trifuoggi M, Giarra A, Bosco L, Montano L, Piscopo M (2020b) Molecular alterations in spermatozoa of a family case living in the land of fires. A First Look at Possible Transgenerational Effects of Pollutants. Int J Mol Sci 21(18). https://doi.org/10.3390/ijms21186710
Li Y, Seto E (2016) HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 6(10). https://doi.org/10.1101/cshperspect.a026831
Li TT, Ogino S, Qian ZR (2014) Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol 20(47):17699–17708. https://doi.org/10.3748/wjg.v20.i47.17699
Li H, Krstin S, Wang S, Wink M (2018) Capsaicin and Piperine can overcome multidrug resistance in cancer cells to doxorubicin. Molecules 23(3). https://doi.org/10.3390/molecules23030557
Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Koskenvuo JK, M E, Pukkala AS, Hemminki K (2000) Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343(2):78–85
Liu NC, Hsieh PF, Hsieh MK, Zeng ZM, Cheng HL, Liao JW, Chueh PJ (2012) Capsaicin-mediated tNOX (ENOX2) up-regulation enhances cell proliferation and migration in vitro and in vivo. J Agric Food Chem 60(10):2758–2765. https://doi.org/10.1021/jf204869w
Liu Z, Zhu P, Tao Y, Shen C, Wang S, Zhao L, Wu H, Fan F, Lin C, Chen C, Zhu Z, Wei Z, Sun L, Liu Y, Wang A, Lu Y (2015) Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem Toxicol 81:1–8. https://doi.org/10.1016/j.fct.2015.04.002
Liu C, Chen C, Yang F, Li X, Cheng L, Song Y (2018) Phytic acid improves intestinal mucosal barrier damage and reduces serum levels of proinflammatory cytokines in a 1,2-dimethylhydrazine-induced rat colorectal cancer model. Br J Nutr 120(2):121–130. https://doi.org/10.1017/S0007114518001290
Lu HF, Chen YL, Yang JS, Yang YY, Liu JY, Hsu SC, Lai KC, Chung JG (2010) Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J Agric Food Chem 58(24):12999–13005. https://doi.org/10.1021/jf103335w
Manuyakorn A, Paulus R, Farrell J, Dawson NA, Tze S, Cheung-Lau G, Hines OJ, Reber H, Seligson DB, Horvath S, Kurdistani SK, Guha C, Dawson DW (2010) Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704. J Clin Oncol 28(8):1358–1365. https://doi.org/10.1200/JCO.2009.24.5639
Michal Freedman D, Kitahara CM, Linet MS, Alexander BH, Neta G, Little MP, Cahoon EK (2015) Ambient temperature and risk of first primary basal cell carcinoma: a nationwide United States cohort study. J Photochem Photobiol B 148:284–289. https://doi.org/10.1016/j.jphotobiol.2015.04.025
Monteleone G, Pallone F, Stolfi C (2012) The dual role of inflammation in colon carcinogenesis. Int J Mol Sci 13(9):11071–11084. https://doi.org/10.3390/ijms130911071
Moradi-Marjaneh R, Hassanian SM, Fiuji H, Soleimanpour S, Ferns GA, Avan A, Khazaei M (2018) Toll like receptor signaling pathway as a potential therapeutic target in colorectal cancer. J Cell Physiol 233(8):5613–5622. https://doi.org/10.1002/jcp.26273
Nalinia N, Sabithaa K, Viswanathanb P, Menona VP (1998) Influence of spices on the bacterial (enzyme) activity in experimental colon cancer. J Ethnopharmacol 62(1):15–24
Naves ER, de Avila Silva L, Sulpice R, Araujo WL, Nunes-Nesi A, Peres LEP, Zsogon A (2019) Capsaicinoids: pungency beyond capsicum. Trends Plant Sci 24(2):109–120. https://doi.org/10.1016/j.tplants.2018.11.001
Nebbioso A, Tambaro FP, Dell'Aversana C, Altucci L (2018) Cancer epigenetics: moving forward. PLoS Genet 14(6):e1007362. https://doi.org/10.1371/journal.pgen.1007362
Nihon-Yanagi Y, Terai K, Murano T, Matsumoto T, Okazumi S (2012) Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol Immunother 61(1):71–77. https://doi.org/10.1007/s00262-011-1085-4
Nolte T, Brander-Weber P, Dangler C, Deschl U, Elwell MR, Greaves P, Hailey R, Leach MW, Pandiri AR, Rogers A, Shackelford CC, Spencer A, Tanaka T, Ward JM (2016) Nonproliferative and proliferative lesions of the gastrointestinal tract, pancreas and salivary glands of the rat and mouse. J Toxicol Pathol 29(1 Suppl):1S–125S. https://doi.org/10.1293/tox.29.1S
Okugawa Y, Grady WM, Goel A (2015) Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 149(5):1204–1225.e1212. https://doi.org/10.1053/j.gastro.2015.07.011
Schulz SF (2006) Methylation of endogenous human retroelements in health and disease. Curr Top Microbiol Immunol 310:211–250
Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK (2005) Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435(7046):1262–1266. https://doi.org/10.1038/nature03672
Seligson DB, Horvath S, McBrian MA, Mah V, Yu H, Tze S et al (2009) Global levels of histone modifications predict prognosis in different cancers. Biomarkers, Genomics, Proteomics, and Gene Regulation 175(5):1619–1628. https://doi.org/10.2353/ajpath.2009.080874
Sharma A, Verma HK, Joshi S, Panwar MS, Mandal CC (2015) A link between cold environment and cancer. Tumor Biol 36(8):5953–5964. https://doi.org/10.1007/s13277-015-3270-0)
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
Singh AK, Bishayee A, Pandey AK (2018) Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients 10(6). https://doi.org/10.3390/nu10060731
Slattery ML, Herrick JS, Bondurant KL, Wolff RK (2012) Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int J Cancer 130(12):2974–2980. https://doi.org/10.1002/ijc.26314
Strahl BD, A. C. (2000) The language of covalent histone. Nature 403(6765):41–45
Tabaei S, Tabaee SS (2019) DNA methylation abnormalities in atherosclerosis. Artif Cells Nanomed Biotechnol 47(1):2031–2041. https://doi.org/10.1080/21691401.2019.1617724
Takahashi K, Sugi Y, Hosono A, Kaminogawa S (2009) Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol 183(10):6522–6529. https://doi.org/10.4049/jimmunol.0901271
Tamagawa H, Oshima T, Numata M, Yamamoto N, Shiozawa M, Morinaga S, Nakamura Y, Yoshihara M, Sakuma Y, Kameda Y, Akaike M, Yukawa N, Rino Y, Masuda M, Miyagi Y (2013) Global histone modification of H3K27 correlates with the outcomes in patients with metachronous liver metastasis of colorectal cancer. Eur J Surg Oncol 39(6):655–661. https://doi.org/10.1016/j.ejso.2013.02.023
Vafadar-Isfahani N, Parr C, McMillan LE, Sanner J, Yeo Z, Saddington S, Peacock O, Cruickshanks HA, Meehan RR, Lund JN, Tufarelli C (2017) Decoupling of DNA methylation and activity of intergenic LINE-1 promoters in colorectal cancer. Epigenetics 12(6):465–475. https://doi.org/10.1080/15592294.2017.1300729
Voskarides K (2019) The “cancer-cold” hypothesis and possible extensions for the Nordic populations. Scand J Public Health 47(5):477–481. https://doi.org/10.1177/1403494819831905
Wei H, Zhang R, Su Y, Bi Y, Li X, Zhang X, Li J, Bao J (2018) Effects of acute cold stress after long-term cold stimulation on antioxidant status, heat shock proteins, inflammation and immune cytokines in broiler heart. Front Physiol 9:1589. https://doi.org/10.3389/fphys.2018.01589
Weichert W, Roske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, Gekeler V, Boehm M, Beckers T, Denkert C (2008) Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res 14(6):1669–1677. https://doi.org/10.1158/1078-0432.CCR-07-0990
Xiang L, Wang S, Jin X, Duan W, Ding X, Zheng C (2012) Expression of BMP2, TLR3, TLR4 and COX2 in colorectal polyps, adenoma and adenocarcinoma. Mol Med Rep 6(5):973–976. https://doi.org/10.3892/mmr.2012.1046
Xie Z, Huang G, Wang Z, Luo S, Zheng P, Zhou Z (2018) Epigenetic regulation of Toll-like receptors and its roles in type 1 diabetes. J Mol Med (Berl) 96(8):741–751. https://doi.org/10.1007/s00109-018-1660-7
Xu H, Wu Q, Dang S, Jin M, Xu J, Cheng Y, Pan M, Wu Y, Zhang C, Zhang Y (2011) Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma. PLoS One 6(12):e27399. https://doi.org/10.1371/journal.pone.0027399
Yang J, Li TZ, Xu GH, Luo BB, Chen YX, Zhang T (2013) Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 60(4):364–372. https://doi.org/10.4149/neo_2013_048
Yoder JA, W a C, Bestor TH (1997) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet 13(8):335–340
Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB (2016) Spices for prevention and treatment of cancers. Nutrients 8(8). https://doi.org/10.3390/nu8080495
Zhivotovskiy AS, Kutikhin AG, Azanov AZ, Yuzhalin AE, Magarill YA, Brusina EB (2012) Colorectal cancer risk factors among the population of South-East Siberia: a case-control study. Asian Pac J Cancer Prev 13(10):5183–5188. https://doi.org/10.7314/apjcp.2012.13.10.5183
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81673944).
Author information
Authors and Affiliations
Contributions
Jingchun Qin and Huixuan Li wrote the first draft of the manuscript. Bin Wen designed the study. Jingchun Qin, Huixuan Li, and Weitao Yu conducted the experiment and analyzed the data. Li Wei contributed to review and edit the paper. Bin Wen revised the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institution’s Ethics Review Board (S2017037).
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, J., Li, H., Yu, W. et al. Effect of cold exposure and capsaicin on the expression of histone acetylation and Toll-like receptors in 1,2-dimethylhydrazine-induced colon carcinogenesis. Environ Sci Pollut Res 28, 60981–60992 (2021). https://doi.org/10.1007/s11356-021-14849-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14849-8