Abstract
A new series of ( ±)-(3-(3,5-dimethyl-1H-pyrazol-1-yl)-6-phenyl-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanones were efficiently synthesized starting from 4-amino-5-hydrazinyl-4H-1,2,4-triazole-3-thiol 1, acetyl acetone 2, various aromatic and heterocyclic aldehydes 3 and phenacyl bromides 4. All the newly synthesized compounds were tested for their antiviral and antitumoral activity. It was shown that subtle structural variations on the phenyl moiety allowed to tune biological properties toward antiviral or antitumoral activity. Mode-of-action studies revealed that the antitumoral activity was due to inhibition of tubulin polymerization.
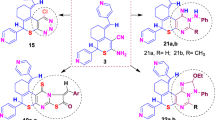
Graphic abstract

Similar content being viewed by others
Introduction
Heterocyclic structures are well-known components of various biologically active compounds. Nitrogen-containing hetero aromatics [1,2,3,4,5,6,7], such as triazole and pyrazole are well known to impart biological activity. Examples of marketed drugs based on a 1,2,4-triazole scaffold include voriconazole (an antifungal drug), forasartan (used for the treatment of hypertension), sitagliptin (an antidiabetic drug) and letrozole (a non-steroidal aromatase inhibitor for the treatment of breast cancer) (Fig. 1) [8]. In addition, a wide range of 1,2,4-triazole derivatives have been synthesized and tested in a wide variety of biological assays, leading to the discovery of anti-bacterial [9, 10], antiviral [11, 12], antifungal [13, 14], anti-inflammatory [15, 16], anti-proliferative [17, 18], anti-convulsant [19], anti-oxidant [20] and anti-Parkinson [21] triazole analogues. Pyrazole ring is another example of a hetero aromatic scaffold, exhibiting a wide range of biological properties. Examples of drugs based on a pyrazole scaffold that received marketing include celecoxib and deracoxib (both cyclo-oxygenase-2 inhibitors), surinabant (a cannabinoid receptor type 1 antagonist) and crizotinib (an ALK inhibitor). However, a plethora of other activities, such as anti-HIV [22, 23], anti-malarial [24], anti-oxidant [25], anti-inflammatory [26], anti-bacterial [27, 28], anti-tumor [29], anti-pyretic [30], anti-analgesic [31], anti-cancer [32] and anti-leishmanial [33] activities have been associated with the pyrazole scaffold.
Although sulfur-containing heterocyclic compounds were found to have extensive biological applications, 1,3,4-thiadiazines are explored to a much lesser extent in medicinal chemistry, when compared to 1,2,4-triazole and pyrazole motifs. Thiadiazines are themselves showing good biological activities [34,35,36,37,38,39].
Multi-component reactions (MCRs), also known as multi-component assembly processes (MCAPs), are attractive synthetic methodologies in medicinal chemistry. The synthetic procedures in MCRs use mild reaction conditions and all, or most, of the atoms from the various reactants contribute to formation of the target compounds. The main advantages of MCRs are their atom economy, eco-friendliness and the fact that it allows to quickly generate structural diversity [40,41,42,43,44].
We recently reported the synthesis of [1, 2, 4]triazolo[3,4-b][1,3,4]thiadiazines through the multi-component reaction (MCR) process [45]. The presence of a hydrazino group in these molecules offers the possibility to convert them into pyrazole moieties. In view of the numerous biological applications of triazoles, pyrazoles and thiadiazines we became interested in the synthesis of the title compounds. Final compounds were subjected to a variety of assays in order to find antiviral and/or antitumoral activity.
Results and discussions
The synthesis of the ( ±)-3-(1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine derivatives was performed using a two-step, one pot procedure. In order to optimize the chemistry, a model reaction was carried out using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole 1, acetylacetone 2, 2,3-dimethoxybenzaldehyde 3 and 4-methoxyphenacylbromide 4 as starting materials (Scheme 1). The first step of the reaction was carried out in ethanol as solvent at reflux temperature, in the presence of a catalytic amount of HCl yielding the intermediate 5-(3,5-dimethyl-1H-pyrazol-1-yl)-4-((4-methoxybenzylidene)amino)-4H-1,2,4-triazole-3-thiol [46]. The intermediate was not isolated instead of it 4-methoxyphenacylbromide 4 was added to the reaction mixture. In order to drive the ring closure to form the thiadiazine moiety, various reaction conditions were explored (Table 1). Running this reaction, either at room temperature or at reflux temperature failed to yield the desired product. Upon the addition of an organic base (such as Pyridine, Piperidine or Triethylamine), the desired product was formed. Using triethylamine as base and running the reaction at reflux temperature (entry 4) resulted in the formation of desired compound ( ±)-5a in excellent yield (Table 1).
Using this methodology (Scheme 2), a series of compounds was prepared using various benzaldehydes, heterocyclic aldehydes and phenacyl bromides (Table 2). This approach is simple and affords the desired products in yields ranging from 83 to 94%. (Table 3).
In the present investigation, pyrazole and dihydrothiadiazine skeletons were developed using one-pot, four-component reaction. Initially, hydrazino functional group of compound 1 underwent cyclocondensation with acetylacetone 2 to form pyrazole ring [47]. Then an appropriate amount of different aldehydes 3 and substituted phenacyl bromides 4 were reacted with amine (–NH2) and thiol (–SH) groups of compound 1 respectively by using triethylamine to establish the dihydrothiadiazines (Scheme 3) [48].
The structures of the final products were confirmed by their spectral data. The FT-IR spectrum of product ( ±)-5b showed a characteristic stretching band at 1681 cm−1 corresponding to the –C=O functional group, whereas the –NH– group appeared at 3135 cm−1. The 1H-NMR spectrum of compound ( ±)-5b showed characteristic peaks, such as two singlets at 2.21 and 2.95 ppm, arising from the two methyl groups on the pyrazole ring. Another two singlets appeared at 2.37 and 2.43 ppm that were assigned to the methyl groups on both phenyl moieties. The two –CH– protons of the dihydrothiadiazine skeleton were visible as two doublets at 5.05 and 5.25 ppm, respectively. The proton of the pyrazole ring showed up as a singlet at 6.00 ppm, whereas the –NH– proton appeared at 7.42 ppm. The remaining aromatic protons appeared in the region of 7.11–7.80 ppm. The 13C-NMR spectrum of compound ( ±)-5b showed peaks at 11.9 and 13.6 ppm for the carbon atoms of two methyl groups on the pyrazole ring at 21.1 and 21.8 ppm for the carbons of two methyl groups on the phenyl moiety. The characteristic carbons of the dihydrothiadiazine skeleton appeared at 44.2 and 59.3 ppm respectively. The pyrazole carbon displayed a peak at 107.8 ppm, whereas the carbonyl peak appeared as the most downfield signal at 193.7 ppm. The remaining aromatic carbons appeared in the range of 127.3 to 151.8 ppm. Mass spectral analysis of compound ( ±)-5b showed a molecular ion peak at m/z 445.
X-ray crystallography
To confirm the structure, crystalline material of compound ( ±)-5 h was isolated, and single crystal X-ray diffraction data were obtained. The compound crystallizes in a monoclinic P21/n space group. The molecular structure of ( ±)–5 h in ORTEP representation is shown in Fig. 2.
Compound ( ±)–5 h has a 4-methylbenzoyl group and 4-chlorophenyl group on two adjacent chiral centers of the six-membered dihydrothiadiazine ring. The dihydrothiadiazine moiety is fused with a triazole ring, further connected to a pyrazole ring through a carbon–nitrogen single bond. The phenyl rings of 4-methylbenzoyl and 4-chlorophenyl groups are almost perpendicular (79.92° and 82.28° respectively) to the mean plane of the fused six- and five-membered rings. The pyrazole ring attached to triazole makes an angle of 55.59° with the mean plane of the fused six- and five-membered rings. The bond distances and angles are consistent with the structure derived from NMR data. The centrosymmetric space group (P21/n) indicates (Table 4) that the material is a racemic mixture. The unit cell contains two pairs of enantiomers and is connected through non-covalent interactions.
Non-covalent intermolecular interactions, such as hydrogen bonding, play an essential role in binding of drugs to their targets, such as DNA or proteins. In this context, the possibility of the presence of non-covalent interactions in the solid state structure of compound ( ±)–5 h was explored. As a result, we were able to identify one N–H … N hydrogen bonding, one C–H … O interaction and one C–H … N interaction (Fig. 3). The interactions and corresponding symmetry transformations are listed in Table 3.
Biological evaluation
In vitro antiviral screening
Compounds (( ±)–5a-t) were subjected to a broad antiviral screening. At a concentration of 100 µM, no selective antiviral activity was observed for the following viruses: influenza A (H1N1 and H3N2) and influenza B virus (in MDCK cells), respiratory syncytial virus (in HEp-2cells), yellow fever virus (in Huh7 cells), herpes simplex virus type 1 and 2 (in HEL 299 cells). However, a number of derivatives did show antiviral activity against the human corona virus 229E (hCoV-229E) in HEL 299 cells (Table 5). Especially compounds ( ±)–5b and ( ±)–5f displayed promising activity with EC50 values of 4.7 and 3.2 µM, respectively. In addition, both derivatives lacked cytotoxicity for the HEL cells giving rise to favorable selectivity indexes.
In vitro antitumoral screening
To investigate their anti-cancer potential, compounds 5a-t were tested in vitro for their anti-proliferative properties, using a real-time IncuCyteproliferation assay against an array of solid and hematological cancers including LN-229 (glioblastoma), Capan-1 (pancreatic adenocarcinoma), HCT-116 (colorectal carcinoma), NCI-H460 (lung carcinoma), DND-41 (acute lymphoblastic leukemia), HL-60 (acute myeloid leukemia), K-562 (chronic myeloid leukemia) and Z-138 (non-Hodgkin lymphoma) cell lines. Docetaxel (a microtubule depolymerisation inhibitor) and staurosporine (STS, a pan-kinase inhibitor) were used as positive controls. From this screening campaign, two derivatives (compounds 5j and 5q) emerged that showed low µM activity against the different cell lines (Table 6).
Because of the promising antitumoral profile of compounds ( ±)–5j and ( ±)–5q, their apoptogenic potential in non-cancerous peripheral blood mononuclear cells (PBMCs) was determined as counter screening. The activation of the executioner caspases-3 and -7 normally precedes the manifestation of apoptosis as massive DNA fragmentation. Therefore, the caspase-3/7 Green reagent was added to the PBMCs, which are also treated with different concentrations of compounds ( ±)–5j and ( ±)–5q. When activated caspase 3 or 7 are intracellularly present, they will cleave the Caspase-3/7 Green Reagent at the DEVD motif. This results in the release of a DNA binding dye that fluorescently labels nuclear DNA of apoptic cells. In addition, in order to distinguish dead cells from live cells, a propidium iodide (PI) staining was carried out. As can be derived from Fig. 4, only very high concentrations of compounds ( ±)–5j and ( ±)–5q (100 µM) give rise to a small increase in the number of apoptotic and dead cells. Overall, these data indicate that compounds ( ±)–5j and ( ±)–5q did not inhibit the viability of normal PBMCs and demonstrate selectivity toward cancer cells over normal cells (Fig. 4).
Despite their promising antitumoral profile, the exact molecular target of compounds ( ±)-5j and ( ±)-5q remained elusive. In order to assess whether they interact with tubulin, an immune fluorescence analysis of tubulin in HEp-2 cells treated for 3 h with compounds ( ±)-5j and ( ±)-5q was performed, and compared to DMSO (vehicle control) and to vincristine (a known tubulin polymerization inhibitor, used as positive control). It can be clearly observed that both compounds ( ±)-5j and ( ±)-5q inhibit the polymerization of tubulin in a dose-dependent manner (Fig. 5).
Immune fluorescence staining of alpha-tubulin in HEp-2 cells: a Representative images of normal alpha-tubulin after treatment with DMSO (top) or typical phenotype after treatment with vincristine (bottom), b Treatment with compounds ( ±)–5j and ( ±)–5q. Green: alpha-tubulin, blue: DAPI. Scale bar: 25 µM
Conclusion
The synthesis of a new series of ( ±)-3-(1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine derivatives was carried out in an excellent yields via a one-pot, four-component method using readily available starting materials. The reactions proceeds in such a way with high atom economy, leading to the formation of one C=N, two C–N, one C–C, and one C–S bonds in a single operation, giving multi-annulated products. All the final compounds were tested for their antiviral and antitumoral activity. It was demonstrated that subtle structural modifications on the phenyl moieties allowed to tune the biological properties of the compounds. Among the newly synthesized compounds, a number of derivatives show promising antiviral activity against the hCoV-229E, whereas other derivatives exhibited cytotoxicity in various cancer cell lines. In addition, it was demonstrated that the antitumoral activity of these compounds is caused by inhibition of tubulin polymerization.
Experimental
General
All the reactants, reagents and solvents were pure, purchased from commercial sources and used without further purification. All the synthesized compounds were preliminarily confirmed by monitoring using TLC plates (E, Merck, Mumbai, India) in the UV-light chamber. A “Stuart SMP30” programmable melting point instrument (Bibby Scientific Ltd. U.K.) was used to record the melting points of the synthesized compounds. FT-IR spectra of the newly synthesized compounds in KBr-pellets were recorded on a PerkinElmer 100S FT-IR spectrophotometer. The 1H- and the 13C-NMR chemical shift values were determined for the compounds on Avance-III Bruker WM-400 MHz spectrometer in δ ppm. Tetramethylsilane (TMS) acts as reference standard for the chemical shifts. Suitable deuterated solvents like CDCl3 and DMSO-d6 were used as solvent for the various compounds to record 1H- and 13C-NMR spectra. Molecular ion peaks were recorded as m/z, ESI-Mass spectra on a PerkinElmer spectrometer performing at 12.5 eV. Carlo Erba EA 1108 CHNS-O automatic analyzer was used for the elemental analysis.
General procedure for the synthesis of ( ±)-(3-(3,5-dimethyl-1H-pyrazol-1-yl)-6-phenyl-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanones (5a-t).
A mixture of 4-amino-5-hydrazino-4H-[1, 2, 4] triazole-3-thiol 1 (1 mmol), acetyl acetone (ACAC) 2 (1 mmol) and appropriate aromatic aldehydes/heterocyclic aldehydes 3 (1 mmol) was taken sequentially in 5 mL of dry ethanol containing drop of Conc. HCl. The reaction mixture was refluxed for 5–7 h by monitoring TLC. After completion of reaction, to the reaction mixture substituted phenacyl bromides 4 (1 mmol) and triethylamine (TEA) (3 mmol) were added and one drop of HCl was neutralized by one mole of TEA. Then the reaction was continued under the reflux for 6–8 h by monitoring TLC (CHCl3:CH3OH = 95:5). The reaction mixture was cooled to room temperature, diluted with water and the solid separated was filtered. The final products were recrystallized from 6–8 mL ethanol.
( ±)-(6-(2,3-Dimethoxyphenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H[1,2,4]triazole [3,4-b][1,3,4]thiadiazin-7-yl)(4-methoxyphenyl)methanone (5a)
Light yellow color solid; yield 92%; m.p.: 192–194 °C; IR (KBr, ʋmax/cm−1): 3211 (NH), 1668 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.24 (s, 3H, CH3), 2.40 (s, 3H, CH3), 3.87 (s, 6H, OCH3), 3.90 (s, 3H, OCH3), 5.28 (unresolved doublet, 2H, CH), 6.00 (s, 1H, CH of pyrazole ring), 6.68 (d, 1H, J = 7.6 Hz, Ar–H), 6.88 (d, 1H, J = 8.0 Hz, Ar–H), 6.94 (s, 1H, NH), 6.97 (d, 2H, J = 8.4 Hz, Ar–H), 7.11 (d, 1H, J = 8.0 Hz, Ar–H), 7.94 (d, 2H, J = 8.8 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.7, 13.7, 42.6, 54.9, 55.7, 55.8, 61.0, 107.7, 112.9, 114.3, 119.7, 124.4, 127.4, 129.4, 130.3, 130.8, 131.2, 142.9, 146.1, 151.8, 152.6, 164.6, 193.2; ESI–MS m/z: 507 [M + H]+; Analytical calculated formulae C25H26N6O4S: C, 59.27; H, 5.17; N, 16.59; S, 6.33; Found: C, 59.22; H, 5.22; N, 16.53; S, 6.30.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(p-tolyl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(p-tolyl)methanone (5b)
White solid; yield 91%; m.p.: 194–196 °C; IR (KBr, ʋmax/cm−1): 3135 (NH), 1681 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.22 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.38 (s, 3H, CH3), 2.43 (s, 3H, CH3), 5.05 (unresolved doublet, 1H, CH), 5.25 (d, 1H, J = 5.2 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 7.11 (d, 2H, J = 8.0 Hz, Ar–H), 7.29 (d, 4H, J = 7.2 Hz, Ar–H), 7.42 (s, 1H, NH), 7.80 (d, 2H, J = 8.0 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.6, 21.1, 21.8, 44.2, 59.3, 107.8, 127.3, 128.8, 129.7, 129.8, 132.0, 132.7, 138.8, 141.3, 143.1, 145.6, 145.7, 151.8, 193.7; ESI–MS m/z: 445 [M + H]+; Analytical calculated formulae C24H24N6OS: C, 64.84; H, 5.44; N, 18.90; S, 7.21; Found: C, 64.89; H, 5.40; N, 18.85; S, 7.18.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(4-nitrophenyl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-nitrophenyl)methanone (5c)
Yellow solid; yield 83%; m.p.: 242–244 °C; IR (KBr, ʋmax/cm−1): 3302 (NH), 1614 (–C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.16 (s, 3H, CH3), 2.44 (s, 3H, CH3), 5.05 (d, 1H, J = 6.0 Hz, CH), 5.20 (d, 1H, J = 6.0 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 7.30 (d, 2H, J = 8.4 Hz, Ar–H), 7.38 (d, 2H, J = 8.4 Hz, Ar–H), 7.65 (d, 2H, J = 8.1 Hz, Ar–H), 7.73 (s, 1H, NH), 7.77 (d, 2H, J = 8.4 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 12.0, 13.5, 44.1, 59.1, 107.9, 128.9, 129.0, 129.2, 129.9, 131.9, 134.3, 134.9, 141.0, 143.1, 145.5, 146.0, 151.8, 193.5; ESI–MS m/z: 507 [M + H]+; Analytical calculated formulae C22H18N8O5S: C, 52.17; H, 3.58; N, 22.12; S, 6.33; Found: C, 52.23; H, 3.54; N, 22.17; S, 6.30.
( ±)-(4-Chlorophenyl)(3-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(3-methoxyphenyl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4][1,3,4]thiadiazin-7-yl)methanone (5d)
Cream color solid; yield 86%; m.p.: 188–190 °C; IR (KBr, ʋmax/cm−1): 3138 (NH), 1692 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.19 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 5.04 (t, 1H, J = 4.0 Hz, CH), 5.25 (d, 1H, J = 4.4 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 6.83 (d, 1H, J = 6.8 Hz, Ar–H), 6.96 (s, 1H, Ar–H), 6.98 (d, 1H, J = 6.4 Hz, Ar–H), 7.23 (t, 1H, J = 6.4 Hz, Ar–H), 7.47 (d, 2H, J = 6.4 Hz, Ar–H), 7.58 (s, 1H, NH), 7.84 (d, 2H, J = 6.8 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 12.0, 13.6, 44.9, 55.3, 59.8, 107.9, 113.3, 114.4, 119.4, 129.5, 130.1, 130.2, 132.9, 137.1, 140.9, 141.2, 143.2, 145.5, 151.8, 160.0 192.9; ESI–MS m/z: 481 [M + H]+; Analytical calculated formulae C23H21ClN6O2S: C, 57.44; H, 4.40; N, 17.47; S, 6.67; Found: C, 57.48; H, 4.45; N, 17.42; S, 6.62.
( ±)-(6-(2-Bromophenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(4-fluorophenyl)methanone (5e)
Golden yellow color solid; yield 90%; m.p.: 195–197 °C; IR (KBr, ʋmax/cm−1): 3138 (NH), 1692 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.15 (s, 3H, CH3), 2.48 (s, 3H, CH3), 5.22 (d, 1H, J = 4.0 Hz, CH), 5.50 (t, 1H, J = 4.8 Hz, CH), 5.99 (s, 1H, CH of pyrazole ring), 7.17 (s, 1H, NH), 7.20 (d, 2H, J = 8.4 Hz, Ar–H), 7.24 (d, 1H, J = 2.0 Hz, Ar–H), 7.59 (d, 1H, J = 7.6 Hz, Ar–H), 7.78 (d, 1H, J = 5.6 Hz, Ar–H), 8.01 (dd, 2H, J = 8.8 Hz, J = 5,2 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.3, 43.1, 58.3, 107.8, 116.3, 116.5, 123.2, 128.1, 128.6, 130.2, 131.7, 131.8, 133.4, 136.4, 140.0, 142.9, 145.5, 151.7, 166.2, 167.8 192.5; ESI–MS m/z: 515 [M + 2]+; Analytical calculated formulae C22H18BrFN6OS: C, 51.47; H, 3.53; N, 16.37; S, 6.25; Found: C, 51.42; H, 3.57; N, 16.32; S, 6.20.
( ±)-(6-(2-Bromophenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(p-tolyl)methanone (5f)
Lemon yellow color solid; yield 92%; m.p.: 201–203 °C; IR (KBr, ʋmax/cm−1): 3148 (NH), 1673 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.20 (s, 3H, CH3), 2.44 (s, 3H, CH3), 2.46 (s, 3H, CH3), 5.22 (d, 1H, J = 4.0 Hz, CH-), 5.49 (t, 1H, J = 4.8 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 7.19 (t, 2H, J = 8.0 Hz, Ar–H), 7.23 (s, 1H, NH), 7.31 (d, 2H, J = 8.0 Hz, Ar–H), 7.58 (d, 1H, J = 7.6 Hz, Ar–H), 7.65 (d, 1H, J = 5.2 Hz, Ar–H), 7.86 (d, 2H, J = 8.0 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.5, 21.8, 42.9, 57.8, 107.8, 123.0, 128.1, 128.6, 128.9, 129.9, 130.2, 131.7, 133.4, 136.6 140.1, 142.9, 145.6, 145.7, 151.8, 193.7; ESI–MS m/z: 511 [M + 2]+; Analytical calculated formulae C23H21BrN6OS: C, 54.23; H, 4.16; N, 16.50; S, 6.29; Found: C, 54.28; H, 4.21; N, 16.44; S, 6.33.
( ±)-(4-Bromophenyl)(6-(4-chlorophenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H- triazolo[3,4-b][1,3,4]thiadiazin-7-yl)methanone (5g)
White solid; yield 90%; m.p.: 205–207 °C; IR (KBr, ʋmax/cm−1): 3291 (NH), 1688 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.16 (s, 3H, –CH3), 2.44 (s, 3H, CH3), 5.05 (dd, 1H, J = 6.0 Hz, J = 3.6 Hz, CH–), 5.2 (d, 1H, J = 6.0 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 7.30 (d, 2H, J = 8.4 Hz, Ar–H), 7.38 (d, 2H, J = 8.4 Hz, Ar–H), 7.65 (d, 2H, J = 8.1 Hz, Ar–H), 7.72 (s, 1H, NH), 7.77 (d, 2H, J = 8.4 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 12.0, 13.5, 44.9, 59.5, 107.9, 129.0, 129.3, 132.1, 130.2 132.6, 133.2, 134.2, 135.1, 140.6, 143.1, 145.3, 151.8, 192.8; ESI–MS m/z: 531 [M + 2]+; Analytical calculated formulae C22H18BrClN6OS: C, 49.87; H, 3.42; N, 15.86; S, 6.05; Found: C, 49.84; H, 3.48; N, 15.83; S, 6.12.
( ±)-(6-(4-Chlorophenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b] [1,3,4]thiadiazin-7-yl)(p-tolyl)methanone (5 h)
White solid; yield 93%; m.p.: 214–216 °C; IR (KBr, ʋmax/cm−1): 3219 (NH), 1675 (C=O); 1H-NMR (400 MHz, CDCl3 + DMSO-d6, δ ppm): 2.25 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.44 (s, 3H, CH3), 3.21 (s, 1H, NH), 4.99 (unresolved singlet, 1H, CH), 5.69 (d, 1H, J = 5.2 Hz, CH), 6.06 (s, 1H, CH of pyrazole ring), 7.19 (d, 1H, J = 7.2 Hz, Ar–H), 7.28 (d, 1H, J = 6.4 Hz, Ar–H), 7.33 (d, 2H, J = 7.6 Hz, Ar–H), 7.47 (s, 2H, Ar–H), 7.90 (s, 2H, Ar–H); 13C-NMR (100 MHz, CDCl3 + DMSO-d6, δ ppm): 11.4, 13.7, 21.8, 42.5, 58.2, 107.6, 128.9, 129.1, 129.3, 129.8, 132.2, 133.9, 135.2, 141.8, 142.8, 145.6, 146.6, 151.4, 194.3; ESI–MS m/z: 465 [M + H]+; Analytical calculated formulae C23H21ClN6OS: C, 59.41; H, 4.55; N, 18.07; S, 6.90; Found: C, 59.45; H, 4.51; N, 18.10; S, 6.85.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(4-hydroxy-3,5-dimethoxyphenyl)-6,7-dihydro-5H- triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanone (5i)
Green solid; yield 89%; m.p.: 196–198 °C; IR (KBr, ʋmax/cm−1): 3435 (OH), 3134 (NH), 1653 (C=O); 1H-NMR (400 MHz, CDCl3 + DMSO-d6, δ ppm): 2.21 (s, 3H, CH3), 2.25 (s, 3H, CH3), 3.71 (s, 6H, OCH3), 4.80 (t, 1H, J = 6.8 Hz, CH), 5.86 (d, 1H, J = 6.0 Hz, CH), 6.11 (s, 1H, CH of pyrazole ring), 6.35 (s, 1H, OH), 6.77 (s, 2H, Ar–H), 7.07 (s, 1H, NH), 7.54 (t, 2H, J = 8.0 Hz, Ar–H), 7.67 (t, 1H, J = 7.2 Hz, Ar–H), 8.00 (d, 2H, J = 7.2 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.5, 45.4, 56.2, 60.8, 104.8, 107.8, 128.7, 129.1, 131.0, 134.5, 134.7, 138.4, 141.5, 143.2, 145.4, 151.7, 153.5, 194.1; ESI–MS m/z: 493 [M + H]+; Analytical calculated formulae C24H24N6O4S: C, 58.52; H, 4.91; N, 17.06; S, 6.51; Found: C, 58.57; H, 4.94; N, 17.10; S, 6.47.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(3,4,5-trifluorophenyl)-6,7-dihydro-5H-[1,2,4]triazole [3,4-b][1,3,4]thiadiazin-7-yl)(p-tolyl)methanone (5j)
White solid; yield 92%; m.p.: 215–217 °C; IR (KBr, ʋmax/cm−1): 3219 (NH), 1674 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.19 (s, 3H, CH3), 2.43 (s, 3H, CH3), 2.50 (s, 3H, CH3), 3.48 (s, 1H, NH), 5.035 (t, 1H, J = 8.0 Hz, CH), 5.48 (d, 1H, J = 8.0 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 6.67 (t, 2H, J = 8.4 Hz, Ar–H), 7.30 (d, 2H, J = 8.4 Hz, Ar–H), 7.82 (d, 2H, J = 8.0 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.5, 21.8, 44.1, 53.1, 101.3, 107.9, 127.5, 128.9, 129.9, 129.8, 131.6, 141.3, 143.2, 145.6, 146.1, 151.8, 160.0, 192.2; ESI–MS m/z: 485 [M + H]+; Analytical calculated formulae C23H19F3N6OS: C, 57.02; H, 3.95; N, 17.35; S, 6.62; Found: C, 57.17; H, 3.99; N, 17.39; S, 6.62.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(3,4,5-trifluorophenyl)-6,7-dihydro-5H-[1,2,4]triazole [3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanone (5 k)
Light yellow color solid; yield 90%; m.p.: 182–184 °C; IR (KBr, ʋmax/cm−1): 3207 (NH), 1681 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.15 (s, 3H, CH3), 2.49 (s, 3H, CH3), 5.34 (t, 1H, J = 8.0 Hz, CH–), 5.56 (d, 1H, J = 8.4 Hz, CH), 5.99 (s, 1H, CH of pyrazole ring), 6.67 (t, 2H, J = 8.4 Hz, Ar–H), 7.15 (s, 1H, NH), 7.51 (t, 2H, J = 7.6 Hz, Ar–H), 7.64 (t, 1H, J = 7.2 Hz, Ar–H), 7.94 (d, 2H, J = 7.6 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.4, 44.3, 53.2, 101.3, 107.9, 127.5, 128.9, 129.1, 129.2, 130.4, 134.8, 134.9, 141.2, 143.2, 145.6, 151.8, 192.7; ESI–MS m/z: 471 [M + H]+; Analytical calculated formulae C22H17F3N6OS: C, 56.16; H, 3.64; N, 17.86; S, 6.82; Found: C, 56.12; H, 3.60; N, 17.90; S, 6.87.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-triazole[3,4-b][1,3,4]thiadiazin-7-yl)(4-fluorophenyl)methanone (5 l)
White solid; yield 88%; m.p.: 196–198 °C; IR (KBr, ʋmax/cm−1): 3211 (NH), 1668 (C = O); 1H-NMR (400 MHz, CDCl3 + DMSO-d6, δ ppm): 2.18 (s, 3H, CH3), 2.22 (s, 3H, CH3), 3.69 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.45 (t, 1H, J = 6.8 Hz, CH), 5.83 (d, 1H, J = 6.4 Hz, CH), 6.15 (s, 1H, CH of pyrazole ring), 6.87 (s, 1H, Ar–H), 6.97 (s, 1H, Ar–H), 7.07 (s, 1H, NH), 7.12 (d, 2H, J = 7.2 Hz, Ar–H), 8.01 (d, 2H, J = 8.4 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.7, 13.7, 42.5, 54.9, 55.7, 55.8, 61.0, 107.7, 112.9, 114.3, 119.7, 124.4, 127.4, 129.4, 131.2, 142.9, 151.7, 152.6, 164.6, 193.2;ESI–MS m/z: 525 [M + H]+; Analytical calculated formulae C25H25FN6O4S: C, 57.24; H, 4.80; N, 16.02; S, 6.11; Found: C, 57.20; H, 4.85; N, 16.17; S, 6.15.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-[1,2,4]triazole [3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanone (5m)
Golden yellow color solid; yield 94%; m.p.: 192–194 °C; IR (KBr, ʋmax/cm−1): 3129 (NH), 1680 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.19 (s, 3H, CH3), 2.39 (s, 3H, CH3), 3.48 (s, 6H, OCH3), 3.78 (s, 3H, OCH3), 5.00 (t, 1H, J = 5.2 Hz, CH), 5.31 (d, 1H, J = 5.6 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 6.66 (s, 2H, Ar–H), 7.50 (t, 2H, J = 7.6 Hz, Ar–H), 7.59 (s, 1H, NH), 7.63 (t, 1H, J = 7.6 Hz, Ar–H), 7.90 (d, 2H, J = 7.6 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.9, 13.5, 45.4, 56.2, 60.6, 60.8, 104.8, 107.8, 128.7, 129.1, 131.0, 134.5, 134.7, 138.4, 141.5, 143.2, 145.4, 151.7, 153.5, 194.1; ESI–MS m/z: 507 [M + H]+; Analytical calculated formulae C25H26N6O4S: C, 59.27; H, 5.17; N, 16.59; S, 6.33; Found: C, 59.24; H, 5.20; N, 16.54; S, 6.38.
( ±)-(6-(2-Chlorophenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-methoxyphenyl)methanone (5n)
White solid; yield 86%; m.p.: 198–200 °C; IR (KBr, ʋmax/cm−1): 3143 (NH), 1671 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.21 (s, 3H, CH3), 2.45 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 5.18 (d, 1H, J = 4.0 Hz, CH), 5.50 (t, 1H, J = 4.4 Hz, CH), 6.00 (s, 1H, CH of pyrazole ring), 6.98 (d, 2H, J = 8.8 Hz, Ar–H), 7.16–7.23 (m, 2H, Ar–H), 7.29 (s, 1H, NH), 7.40 (d, 1H, J = 8.0 Hz, Ar–H), 7.60 (d, 1H, J = 4.8 Hz, Ar–H), 7.94 (d, 2H, J = 8.4 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 12.0, 13.5, 21.8, 44.1, 59.1, 107.9, 128.9, 129.0, 129.2, 129.9, 131.9, 134.3, 134.9, 141.0, 143.1, 145.5, 145.9, 151.8, 193.5; ESI–MS m/z: 481 [M + H]+; Analytical calculated formulae C23H21ClN6O2S: C, 57.44; H, 4.40; N, 17.47; S, 6.67; Found: C, 57.40; H, 4.45; N, 17.1; S, 6.62.
( ±)-(6-(2,4-Dimethoxyphenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazole [3,4-b][1,3,4]thiadiazin-7-yl)(4-methoxyphenyl)methanone (5o)
Yellow solid; yield 94%; m.p.: 150–152 °C; IR (KBr, ʋmax/cm−1): 3129 (NH), 1680 (C=O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 2.19 (s, 3H, CH3), 2.22 (s, 3H, CH3), 3.69 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.45 (t, 1H, J = 6.8 Hz, CH), 5.83 (d, 1H, J = 6.4 Hz, CH), 6.16 (s, 1H, CH of pyrazole ring), 6.86 (d, 1H, J = 8.4 Hz, Ar–H), 6.98 (d, 1H, J = 8.4 Hz, Ar–H), 7.07 (s, 1H, NH), 7.10 (d, 2H, J = 8.0 Hz, Ar–H), 7.12 (s, 1H, Ar–H), 8.01 (d, 2H, J = 8.4 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.7, 13.7, 42.6, 54.9, 55.7, 55.8, 61.0, 107.7, 112.9, 114.3, 119.7, 124.4, 127.4, 129.4, 130.3, 130.8, 131.2, 142.9, 146.1, 151.8, 152.6, 164.6, 193.2; ESI–MS m/z: 507 [M + H]+; Analytical calculated formulae C25H26N6O4S: C, 59.27; H, 5.17; N, 16.59; S, 6.33; Found: C, 59.24; H, 5.14; N, 16.63; S, 6.30.
( ±)-(6-(2,4-dimethoxyphenyl)-3-(3,5-dimethyl-1H-pyrazol-1-yl)-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-nitrophenyl)methanone (5p)
Yellow solid; yield 88%; m.p.: 236–238 °C; IR (KBr, ʋmax/cm−1): 3135 (NH), 1681 (C=O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.35 (s, 3H, CH3), 2.42 (s, 3H, CH3), 3.79 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.08 (s, 1H, CH of pyrazole ring), 6.48 (s, 1H, Ar–H), 6.62 (dd, 1H, J = 8.4 Hz, J = 6.4 Hz, Ar–H), 7.27 (s, 1H, NH), 7.43 (d, 1H, J = 8.8 Hz, Ar–H), 7.95 (d, 2H, J = 8.8 Hz, Ar–H), 8.31 (d, 2H, J = 8.8 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.7, 13.7, 55.6, 55.7, 98.5, 104.7, 108.1, 112.9, 114.6, 123.9, 131.0, 131.2, 138.3, 141.2, 143.6, 146.5, 149.4, 152.4, 16.2, 159.1, 163.3, 196.0; Analytical calculated formulae C24H21N7O5S: C, 55.48; H, 4.07; N, 18.87; S, 6.17; Found: C, 55.43; H, 4.02; N, 18.92; S, 6.21.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(furan-2-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-methoxyphenyl)methanone (5q)
Brown solid; yield 87%; m.p.: 152–154○C; IR (KBr, ʋmax/cm−1): 3143 (NH), 1671 (C = O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.30 (s, 3H, CH3), 2.34 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 5.23 (unresolved singlet, 2H, CH and CH), 6.03 (s, 1H, CH of pyrazole ring), 6.30 (s, 1H, CH-), 6.32 (s, 1H, CH), 7.02 (d, 3H, J = 8.8 Hz, Ar–H), 7.35 (s, 1H, NH), 7.94 (d, 2H, J = 8.4 Hz, Ar–H); ESI–MS m/z 437 [M + H]+; Analytical calculated formulae C21H20N6O3S: C, 57.79; H, 4.62; N, 19.25; S, 7.35; Found: C, 57.83; H, 4.66; N, 19.21; S, 7.31.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(furan-2-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b] [1,3,4]thiadiazin-7-yl)(p-tolyl)methanone (5r)
Brown solid; yield 85%; m.p.: 149–151○C; IR (KBr, ʋmax/cm−1): 3204 (NH), 1666 (C = O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.30 (s, 3H, CH3), 2.34 (s, 3H, CH3), 2.46 (s, 3H, CH3), 5.26 (unresolved singlet, 2H, CH-), 6.03 (s, 1H, CH of pyrazole ring), 6.31 (d, 2H, J = 8.0 Hz, Ar–H), 7.03 (s, 1H, NH), 7.35 (d, 3H, J = 8.8 Hz, Ar–H), 7.85 (d, 2H, J = 8.0 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.6, 13.7, 21.9, 39.8, 53.0, 107.7, 109.2, 111.0, 128.9, 130.0, 131.6, 140.6, 142.7, 143.1, 146.1, 146.7, 148.3, 152.0, 194.4; ESI–MS m/z: 421 [M + H]+; Analytical calculated formulae C21H20N6O2S: C, 59.98; H, 4.79; N, 19.99; S, 7.63; Found: C, 59.94; H, 4.7; N, 19.94; S, 7.68.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(thiophen-2-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-nitrophenyl)methanone (5s)
Golden color solid; yield 89%; m.p.: 204–206○C; IR (KBr, ʋmax/cm−1): 3281(NH), 1696 (C=O); 1H-NMR (400 MHz, CDCl3 + DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.27 (s, 3H, CH3), 5.47 (t, 1H, J = 3.6 Hz, CH), 5.88 (d, 1H, J = 3.2 Hz, CH), 6.10 (s, 1H, CH of pyrazole ring), 6.97 (t, 1H, J = 4.4 Hz, Ar–H), 7.17 (d, 1H, J = 4.4 Hz, Ar–H), 7.31 (d, 1H, J = 4.8 Hz, Ar–H), 7.35 (s, 1H, NH), 8.37 (s, 4H, Ar–H); 13C-NMR (100 MHz, CDCl3 + DMSO-d6, δ ppm): 11.4, 13.8, 42.9, 53.4, 107.5, 124.1, 126.3, 126.5, 127.4, 129.3, 130.8, 139.9, 142.9, 146.9, 149.6, 150.7, 151.3, 194.5; ESI–MS m/z: 468 [M + H]+; Analytical calculated formulae C20H17N7O3S: C, 51.38; H, 3.67; N, 20.97; S, 13.72; Found: C, 51.34; H, 3.62; N, 20.94; S, 13.76.
( ±)-(3-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(furan-2-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-7-yl)(4-nitrophenyl)methanone (5t)
White solid; yield 92%; m.p.: 201–203○C; IR (KBr, ʋmax/cm−1): 3278 (NH), 1698 (C = O); 1H-NMR (400 MHz, CDCl3, δ ppm): 2.25 (s, 3H, CH3), 2.27 (s, 3H, CH3), 5.28–5.32 (m, 1H, CH), 5.78 (d, 1H, J = 4.0 Hz, CH), 6.08 (s, 1H, CH of pyrazole ring), 6.45 (s, 1H, NH), 7.14 (d, 1H, J = 4.0 Hz, Ar–H), 7.48 (d, 1H, J = 4.0 Hz, Ar–H), 7.85 (t, 1H, J = 8.4 Hz, Ar–H), 8.29 (d, 2H J = 8.4 Hz, Ar–H), 8.33 (d, 2H, J = 7.2 Hz, Ar–H); 13C-NMR (100 MHz, CDCl3, δ ppm): 11.2, 13.8, 49.8, 52.5, 107.5, 109.2, 111.0, 124.1, 124.3, 130.4, 130.6, 142.9, 143.0, 148.1, 149.1, 150.7 151.3, 194.0; ESI–MS m/z: 452 [M + H]+; Analytical calculated formulae C20H17N7O4S: C, 53.21; H, 3.80; N, 21.72; S, 7.10; Found: C, 53.25; H, 3.85; N, 21.7; S, 7.15.
X-ray crystallography
The diffraction data was collected on Bruker APEX2 single crystal X-ray diffractometere quipped with a CCD area detector system, graphite mono chromator and a Mo-Kα fine focus sealed tube (λ = 0.71073 Å). Bruker SAINT PLUS was used for data reduction, SHELXT-2014 [49] was used for structure solution and SHELXL-2018 [50] was used for full-matrix least-squares refinement. Mercury 3.3 [51] was used for molecular graphics. All non-hydrogen atoms were refined using anisotropic thermal parameters. All hydrogen atoms bound to carbons were positioned geometrically and refined using a riding model. Important crystallographic data and table for bond distances and bond angles were provided in supporting information.
References
Wan J-P, Cao S, Liu Y (2016) Base-promoted synthesis of N -substituted 1,2,3-triazoles via enaminone-azide cycloaddition involving regitz diazo transfer. Org Lett 18:6034–6037. https://doi.org/10.1021/acs.orglett.6b02975
Wang X-Y, Zhong Y-F, Mo Z-Y et al (2021) Synthesis of seleno oxindoles via electrochemical cyclization of N -arylacrylamides with diorganyl diselenides. Adv Synth Catal 363:208–214. https://doi.org/10.1002/adsc.202001192
Zhong P-F, Lin H-M, Wang L-W et al (2020) Electrochemically enabled synthesis of sulfide imidazopyridines via a radical cyclization cascade. Green Chem 22:6334–6339. https://doi.org/10.1039/D0GC02125C
Guo Y, Wang G, Wei L, Wan J-P (2019) Domino C-H sulfonylation and pyrazole annulation for fully substituted pyrazole synthesis in water using hydrophilic enaminones. J Org Chem 84:2984–2990. https://doi.org/10.1021/acs.joc.8b02897
Wang M-R, Deng L, Liu G-C et al (2019) Porous organic polymer-derived nanopalladium catalysts for chemoselective synthesis of antitumor benzofuro[2,3- b ]pyrazine from 2-bromophenol and isonitriles. Org Lett 21:4929–4932. https://doi.org/10.1021/acs.orglett.9b01230
Wan J-P, Jing Y, Hu C, Sheng S (2016) Metal-free synthesis of fully substituted pyridines via ring construction based on the domino reactions of enaminones and aldehydes. J Org Chem 81:6826–6831. https://doi.org/10.1021/acs.joc.6b01149
Tong W, Li W-H, He Y et al (2018) Palladium-metalated porous organic polymers as recyclable catalysts for the chemioselective synthesis of thiazoles from thiobenzamides and isonitriles. Org Lett 20:2494–2498. https://doi.org/10.1021/acs.orglett.8b00886
Kaur P, Chawla A (2017) 1,2,4-Triazole: a review of pharmacological activities. Int Res J Pharm 8:10–29. https://doi.org/10.7897/2230-8407.087112
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009) Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem 44:4362–4366. https://doi.org/10.1016/j.ejmech.2009.05.022
Burman WJ (2010) Rip van winkle wakes up: Development of tuberculosis treatment in the 21st century. Clin Infect Dis 50:165–172. https://doi.org/10.1086/651487
Abdel-Aal MT, El-Sayed WA, El-Kosy SM, El-Ashry ESH (2008) Synthesis and antiviral evaluation of novel 5-(N-arylaminomethyl-1,3,4- oxadiazol-2-yl)hydrazines and their sugars, 1,2,4-triazoles, tetrazoles and pyrazolyl derivatives. Arch Pharm (Weinheim) 341:307–313. https://doi.org/10.1002/ardp.200700154
Benci K, Suhina T, Mandić L et al (2011) Novel 1,2,4-triazole and purine acyclic cyclopropane nucleoside analogues: synthesis, antiviral and cytostatic activity evaluations. Antivir Chem Chemother 21:221–230. https://doi.org/10.3851/IMP1762
Rezaei Z, Khabnadideh S, Pakshir K et al (2009) Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2008.07.012
Andrews B, Ahmed M (2014) Synthesis and characterization of pyrimidine bearing 1,2,4-triazole derivatives and their potential antifungal action. Int J ChemTech Res 6:1013–1021
Mathew V, Keshavayya J, Vaidya VP (2006) Heterocyclic system containing bridgehead nitrogen atom: synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur J Med Chem 41:1048–1058. https://doi.org/10.1016/j.ejmech.2006.03.018
Jiang B, Huang X, Yao H et al (2014) Discovery of potential anti-inflammatory drugs: Diaryl-1,2,4-triazoles bearing N-hydroxyurea moiety as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. Org Biomol Chem 12:2114–2127. https://doi.org/10.1039/c3ob41936c
Abuo-Rahma GEDAA, Abdel-Aziz M, Beshr EAM, Ali TFS (2014) 1,2,4-Triazole/oxime hybrids as new strategy for nitric oxide donors: synthesis, anti-inflammatory, ulceroginicity and antiproliferative activities. Eur J Med Chem 71:185–198. https://doi.org/10.1016/j.ejmech.2013.11.006
Shivarama Holla B, Veerendra B, Shivananda M, Poojary B (2003) Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 38:759–767. https://doi.org/10.1016/S0223-5234(03)00128-4
Plech T, Luszczki JJ, Wujec M et al (2013) Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur J Med Chem 60:208–215. https://doi.org/10.1016/j.ejmech.2012.11.026
Cetin A, Geçibesler IH (2015) Evaluation as antioxidant agents of 1,2,4-triazole derivatives: effects of essential functional groups. J Appl Pharm Sci 5:120–126. https://doi.org/10.7324/JAPS.2015.50620
Ongini E, Monopoli A, Cacciari B, Giovanni Baraldi P (2001) Selective adenosine A2A receptor antagonists. Farm 56:87–90. https://doi.org/10.1016/S0014-827X(01)01024-2
Messore A, Corona A, Madia VN et al (2020) Pyrrolyl pyrazoles as non-diketo acid inhibitors of the HIV-1 ribonuclease H function of reverse transcriptase. ACS Med Chem Lett 11:798–805. https://doi.org/10.1021/acsmedchemlett.9b00617
Kim J, Lee D, Park C et al (2012) Discovery of phenylaminopyridine derivatives as novel HIV-1 non-nucleoside reverse transcriptase inhibitors. ACS Med Chem Lett 3:678–682. https://doi.org/10.1021/ml300146q
Jiang B, Fan W, Sun MY et al (2014) Domino reaction of arylglyoxals with pyrazol-5-amines: selective access to pyrazolo-fused 1,7-naphthyridines, 1,3-diazocanes, and pyrroles. J Org Chem 79:5258–5268. https://doi.org/10.1021/jo500823z
Renuka N, Ajay Kumar K (2013) Synthesis and biological evaluation of novel formyl-pyrazoles bearing coumarin moiety as potent antimicrobial and antioxidant agents. Bioorganic Med Chem Lett 23:6406–6409. https://doi.org/10.1016/j.bmcl.2013.09.053
Tewari AK, Srivastava P, Singh VP et al (2010) Novel anti-inflammatory agents based on pyrazole based dimeric compounds; design, synthesis, docking and in vivo activity. Chem Pharm Bull (Tokyo) 58:634–638. https://doi.org/10.1248/cpb.58.634
Vijesh AM, Isloor AM, Shetty P et al (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62:410–415. https://doi.org/10.1016/j.ejmech.2012.12.057
Poce G, Consalvi S, Venditti G et al (2019) Novel pyrazole-containing compounds active against mycobacterium tuberculosis. ACS Med Chem Lett 10:1423–1429. https://doi.org/10.1021/acsmedchemlett.9b00204
Park HJ, Lee K, Park SJ et al (2005) Identification of antitumor activity of pyrazole oxime ethers. Bioorganic Med Chem Lett 15:3307–3312. https://doi.org/10.1016/j.bmcl.2005.03.082
Sener A, Kasimogullari R, Sener MK, Genc H (2004) Studies on reactions of cyclic oxalyl compounds with hydrazines or hydrazones. 2. Synthesis and reactions of 4-benzoyl-1-(4-nitrophenyl)-5-phenyl- 1H-pyrazole-3-carboxylic acid. Chem Heterocycl Compd 40:1039–1046. https://doi.org/10.1023/B:COHC.0000046695.00178.79
Menozzi G, Mosti L, Fossa P et al (1997) ω-Dialkylaminoalkyl ethers of phenyl-(5-substituted 1-phenyl-1 H -pyrazol-4-yl)methanols with analgesic and anti-inflammatory activity. J Heterocycl Chem 34:963–968. https://doi.org/10.1002/jhet.5570340339
Magedov IV, Manpadi M, Van Slambrouck S et al (2007) Discovery and investigation of antiproliferative and apoptosis-inducing properties of new heterocyclic podophyllotoxin analogues accessible by a one-step multicomponent synthesis. J Med Chem 50:5183–5192. https://doi.org/10.1021/jm070528f
Mowbray CE, Braillard S, Speed W et al (2015) Novel amino-pyrazole ureas with potent in vitro and in vivo antileishmanial activity. J Med Chem 58:9615–9624. https://doi.org/10.1021/acs.jmedchem.5b01456
Zhang B, Li Y-H, Liu Y et al (2015) Design, synthesis and biological evaluation of novel 1,2,4-triazolo [3,4-b][1,3,4] thiadiazines bearing furan and thiophene nucleus. Eur J Med Chem 103:335–342. https://doi.org/10.1016/j.ejmech.2015.08.053
Shah TA, Ahmad Z, Mir NA et al (2015) One step synthesis of highly functionalized thiazolo[3{,}2-b][1{,}2{,}4]triazole{,} triazolo[1{,}5-a]pyrimidine and triazolo[3{,}4-b][1{,}3{,}4]thiadiazine. RSC Adv 5:107931–107937. https://doi.org/10.1039/C5RA21270G
Khan I, Ibrar A, Abbas N (2013) Triazolothiadiazoles and triazolothiadiazines – biologically attractive scaffolds. Eur J Med Chem 63:854–868. https://doi.org/10.1016/j.ejmech.2013.01.060
Sumangala V, Poojary B, Chidananda N et al (2012) Facile synthesis, cytotoxic and antimicrobial activity studies of a new group of 6-aryl-3-[4-(methylsulfonyl)benzyl]-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. Eur J Med Chem 54:59–64. https://doi.org/10.1016/j.ejmech.2012.04.024
Khan I, Zaib S, Ibrar A et al (2014) Synthesis, crystal structure and biological evaluation of some novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Eur J Med Chem 78:167–177. https://doi.org/10.1016/j.ejmech.2014.03.046
Pundeer R, Kiran V, Prakash R et al (2012) α, α-Dibromoacetophenones mediated synthesis of some new 7H–7-alkoxy-3-alkyl/phenyl-6-aryl-s-triazolo[3,4-b][1,3,4]thiadiazines and their antimicrobial evaluation. Med Chem Res 21:4043–4052. https://doi.org/10.1007/s00044-011-9945-1
Saha A, Payra S, Banerjee S (2015) One-pot multicomponent synthesis of highly functionalized bio-active pyrano[2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives using ZrO 2 nanoparticles as a reusable catalyst. Green Chem 17:2859–2866. https://doi.org/10.1039/C4GC02420F
Bhunia A, Porwal D, Gonnade RG, Biju AT (2013) Multicomponent reactions involving arynes, quinolines, and aldehydes. Org Lett 15:4620–4623. https://doi.org/10.1021/ol4023134
Cioc RC, Ruijter E, Orru RVA (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16:2958–2975. https://doi.org/10.1039/C4GC00013G
Jilloju PC, Shyam P, Sanjeev A, Vedula RR (2021) Four-component, one–pot synthesis of (E)-N-benzylidene-3-(benzylthio)-5-(3,5-dimethyl-1H-pyrazol-1-yl)-4H-1,2,4-triazol-4-amines and their DNA binding and molecular docking studies. J Mol Struct 1225:129140. https://doi.org/10.1016/j.molstruc.2020.129140
Mamidala S, Peddi SR, Aravilli RK et al (2021) Microwave irradiated one pot, three component synthesis of a new series of hybrid coumarin based thiazoles: Antibacterial evaluation and molecular docking studies. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.129114
Sujatha K, Vedula RR (2019) Multicomponent Efficient synthesis of new [1,2,4]Triazolo[3,4]thiadiazines. J Heterocycl Chem 56:832–838. https://doi.org/10.1002/jhet.3458
Jilloju PC, Vedula RR (2018) A facile one-pot three-component synthesis of benzylideneamino-3,5-dimethyl-1H-pyrazoles. Synth Commun 48:1739–1746. https://doi.org/10.1080/00397911.2018.1458242
Div MC, College SC (1989) 4-Amino-3-pyrazolo-l,2,4-triazoles as antimicrobial agents+. Arch Pharm 66:63–66
Al-Etaibi A, John E, Ibrahim MR et al (2011) Stereoselective synthesis of dihydrothiadiazinoazines and dihydrothiadiazinoazoles and their pyrolytic desulfurization ring contraction. Tetrahedron 67:6259–6274. https://doi.org/10.1016/j.tet.2011.06.034
Sheldrick GM (2015) SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Crystallogr 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Macrae CF, Bruno IJ, Chisholm JA et al (2008) Mercury CSD 2.0 - New features for the visualization and investigation of crystal structures. J Appl Crystallogr 41:466–470. https://doi.org/10.1107/S0021889807067908
Acknowledgements
We are thankful to the Head, Department of Chemistry and Director, National Institute of Technology, Warangal, Telangana, for providing infrastructure facilities and one of the authors P.C. Jilloju is thankful to MHRD government of India, for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jilloju, P.C., Persoons, L., Kurapati, S.K. et al. Discovery of ( ±)-3-(1 H -pyrazol-1-yl)-6,7-dihydro-5 H -[1,2,4]triazolo[3,4- b ][1,3,4] thiadiazine derivatives with promising in vitro anticoronavirus and antitumoral activity . Mol Divers 26, 1357–1371 (2022). https://doi.org/10.1007/s11030-021-10258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10258-8