Abstract
Plant S deficiency is common, but the role of S-containing amino acids such as cysteine in plant S uptake is unknown. We applied 14C-, 35S-, 13C-, and 15N-labelled cysteine to wheat and oilseed rape rhizospheres and traced the plants’ elemental uptake. Both plants absorbed 0.37–0.81% of intact cysteine after 6 h with no further increase after 24 h. They absorbed 1.6–11.5% 35S and 12.3–7.6% 15N from cysteine after 24 h and utilised SO42− as their main S source (75.5–86.4%). Added and naturally occurring cysteine-S contributed 5.6 and 1.1% of total S uptake by wheat and oilseed rape, respectively. Cysteine and inorganic S derived from cysteine contributed 24.5 and 13.6% of uptake for wheat and oilseed rape, respectively, after 24 h. Oilseed rape absorbed ~10-fold more S from cysteine and SO42− than did wheat. The highest absorption of free cysteine should be in the organic-rich soil patches. Soil microorganisms rapidly decomposed cysteine (t1/2 = 1.37 h), and roots absorbed mineralised inorganic N and S. After 15 min, 11.7–14.3% of the 35S-cysteine was retained in the microbial biomass, while 30.2–36.7% of the SO42− was released, suggesting that rapid microbial S immobilisation occurs after cysteine addition. Plants acquire N and S from cysteine via unidirectional soil-to-root nutrient flow, and cysteine is an important S source for plants.
Similar content being viewed by others
Introduction
Sulphur (S) is an essential plant macronutrient. It plays a vital role in numerous metabolic processes such as coenzyme A, biotin, chlorophyll, glutathione, and thiamine biosynthesis (Wyngaard and Cabrera 2015). S deficiency in crops has been reported worldwide as a consequence of recent global reductions in atmospheric sulphur dioxide emissions, the use of low-S or S-free fertilisers, and soil S removal resulting from soil organic matter depletion (Dong et al. 2017; Wyngaard and Cabrera 2015). Organic S (OS) accounts for > 90–95% of all soil S (Kopittke et al. 2016). Nevertheless, most prior studies on plant S nutrition focused only on inorganic soil S and assumed that roots can only absorb inorganic sulphate (Ciaffi et al. 2013; Honsel et al. 2012; Prodhan et al. 2017). Hence, little research has been conducted on complex organic S as a plant S source (Bona and Monteiro 2010).
Soil amino acids are produced by extracellular proteolytic enzymes, root exudation, soil microorganisms, and other processes. Several studies showed that plants absorb small-molecule organic N sources such as amino acids (Ganeteg et al. 2017; Hill et al. 2019b; Hill and Jones 2019; Ma et al. 2017) and quaternary ammonium (Warren 2013). Plants also absorb large-molecule organic N from sources such as nucleic acids and proteins (Paungfoolonhienne et al. 2008, 2010). Moreover, amino acids are highly bioavailable and have high turnover rates (Hill et al. 2011, 2019a; Jones et al. 2018a; Jones and Kielland 2012). Plants may absorb S-containing organic molecules such as cysteine and methionine and utilise the S they contain. However, amino acid content is substantially lower than the inorganic N content in the agricultural soil because of inorganic fertilisation, and amino acid-N plays a limited role in plant nutrition (Näsholm et al. 2009). When the inorganic sulphate content is low in the soil, soluble OS could be an important plant S source. Direct intact cysteine uptake could mitigate the energy expenditure required to convert sulphate to cysteine.
Plant roots must compete with soil microorganisms for amino acids. Soil microorganisms are the predominant soil organic matter consumers (Hill et al. 2013; Kuzyakov and Xu 2013; Näsholm et al. 2009). C and S from amino acids may rapidly undergo several complex reactions such as immobilisation into microbial biomass (MB) and the release of MB-bonded S as sulphate. Some of the intact amino acids and inorganic S derived from amino acids may be absorbed by plant roots (Ma et al. 2020a, 2021) (Fig. 1). The ability of soil microorganisms to decompose OS determines the amount of intact residual OS available to plant roots. Nevertheless, the connection between OS decomposition and root uptake remains to be established. Elucidating soil microbial cysteine decomposition will clarify soil C, N, and S cycles and their relationships with root macronutrient uptake.
Schematic diagram (A) and photograph (B) of rhizosphere cultivation and 14C-tracing methods and simplified model of soil cysteine cycling (C). Wheat and oilseed were sown in rhizotubes with aeration holes. The holes also served as injected labelling material ports. The plants were cultivated for 17 days. Aliquots (1.2 mL) of the S treatments were injected into the rhizosphere at six equidistant points using 200 μL pipettes. Rhizotubes injected with 14C were inserted into the apparatus, and their holes were sealed with silicone rubber to separate soil 14CO2 and plant 14CO2 release. The 14CO2 was absorbed with 2 mL of 1 M NaOH. Possible dispositions of cysteine addition included (1) root uptake as intact molecules; (2) absorption of C in the organic S compound by the roots and its release from the leaves as CO2; (3) immobilisation of C, N, and S in the microbial biomass; (4) release of SO42− and NH4+ by soil microorganisms; (5) absorption of SO42− and NH4+ by the plant roots; (6) release of microbial C as CO2; or (7) adsorption of cysteine and its derivative SO42− to the soil particles
Plant roots interact with soil microorganisms, and complex ecological and biological processes occur in the rhizosphere (Kuzyakov and Blagodatskaya 2015; Wei et al. 2019). Rhizodeposits affect microbial community function and composition (Liu et al. 2019) and promote fast-growing Gram-negative bacteria that can utilise low-molecular-weight organic matter (Dippold et al. 2014). Plant roots may face strong competition from soil microorganisms for organic N and S uptake in the rhizosphere.
To investigate plant OS uptake from the rhizosphere, we selected oilseed rape (Brassica campestris L.) and wheat (Triticum aestivum L.) for examination. These crop species markedly differ in terms of their S requirements. For optimum growth, wheat requires 15–20 kg S ha−1, while oilseed rape needs 30–40 kg S ha−1 (Vong et al. 2004). In the present study, we focused on cysteine, as it is the central metabolite coordinating S, C, and N flux in all chemoautotrophic and photoautotrophic organisms (Planta et al. 2017) and is usually present in various soil types (Cao et al. 2016). We performed 13C, 15N, 14C, and 35S label uptake assays on wheat and oilseed rape cultivated in soil in rhizotubes. We used dual 13C and 15N labelling to distinguish intact amino acid absorption from the uptake of N derived from mineralised amino acids (Ganeteg et al. 2017). We also tested for linear correlations between 14C and 35S absorption. Radioisotopes are easily and precisely detected and measured. Moreover, foliar 14CO2 release is simply and rapidly detected. Therefore, radiolabel assays were performed to evaluate the root cysteine-S/sulphate-S uptake ratios after mineralisation.
Soil available N content may also influence the competition for OS between roots and soil microorganisms by modulating microbial and plant element requirements. Hence, we explored how soil available N status affects plants utilising and microorganisms decomposing cysteine. We postulated that (1) roots have access to soil cysteine, but soil microorganisms strongly compete for it; (2) soil microorganisms rapidly decompose cysteine, and mineralised N and S may be available to plants; and (3) relative differences in plant S demand and soil available N content may reflect trends in plant preferences for organic and inorganic S.
Materials and methods
Plant uptake of intact OS from the rhizosphere
Agricultural brown earth soil (FAO classification: Eutric Cambisol) was sampled at a 0–10 cm depth at Henfaes Agricultural Research Station, Abergwyngregyn, Bangor, UK (53° 14′ N, 4° 01′ W). In the laboratory, vegetation, stones, and earthworms were removed from the samples, which were then air-dried to 20% water content and passed through a 2-mm sieve. Basic soil properties were determined using previously reported methods (Hill et al. 2013). The pH was 6.5; the total C and N concentrations were 34 mg g−1 dry soil and 0.54 mg g−1 dry soil, respectively; the total S content was 456 mg kg−1; the soil contained 48.2% sand, 33.6% silt, and 18.2% clay; the soil solution amino acid concentration was 42 μM N; and the soil solution concentration of peptides < 1 kDa was 107 μM N.
Oilseed rape and wheat seeds were maintained in culture dishes for 2 days. Germinated seeds were individually sown in rhizotubes (length: 240 mm; internal diameter: 8 mm) containing 12.5 g agricultural brown earth soil (Fig. 1) (Hill et al. 2013). Aeration holes were cut into the rhizotubes at 1-cm intervals and served as injected labelling material ports. The plants were grown at 15 °C under a 16 h light/8 h dark photoperiod, 500 μmol m−2 s−1 light intensity, and 70% relative humidity for 17 days. Mean root dry weights were 24.9 ± 0.90 mg for wheat and 8.6 ± 0.4 mg for oilseed rape (n = 75). The wheat and oilseed rape roots filled the rhizotubes, and the soil around the roots was considered the rhizosphere. After 17 days, 1.2 mL of each S-treatment was injected at six equidistant points into the rhizosphere using a 200 μL pipette. In this way, the labelled materials could rapidly and uniformly disperse throughout the rhizotubes. We tested this method by adding the blue ink to the rhizotube in the same way as for the labelled solution, and all the soil in the tube turned blue in seconds. The injected solutions included 50 μM 35S-13C-15N-Cys, 50 μM 14C-Cys, 50 μM 35S-13C-15N-Cys + 15 mg N kg−1 dry soil, 50 μM 14C-Cys + 15 mg N kg−1 dry soil, and 50 μM 35S-NaSO4 (35S: 8.9 kBq mL−1; 14C: 5.1 kBq mL−1; L-13C3-15N-Cys, 99.88%; Sigma-Aldrich Corp., St. Louis, MO, USA). Rhizotubes injected with 14C were inserted into the apparatus shown in Fig. 1. The holes in the apparatus cap were sealed with silicone rubber to separate 14CO2 released by the soil and plants. The 14CO2 was absorbed using 2 mL of 1 M NaOH. The plants were destructively harvested after 15 min, 6 h, and 24 h uptake by vertically splitting the rhizotubes with a razor blade.
The soluble SO42− and cysteine concentrations were measured before the injection of the labelled solutions. Five grammes of rhizosphere soil was extracted for 30 min in 25 mL purified water in a flask on a reciprocal shaker at 20 °C and 180 rpm. The extracts were centrifuged at 12,000×g for 10 min at 20 °C, and the SO42− content was evaluated using ion chromatography (930 Compact IC Flex; Metrohm Ltd., Runcorn, UK). To measure the cysteine content in 5 mL supernatant, the protein was first precipitated with sulphosalicylic acid (Ozols 1990). The mixture was centrifuged at 5000×g at 20 °C for 5 min. The cysteine content was measured with an automatic amino acid analyser (L-8900; Hitachi, Chiyoda, Japan).
Five plants were harvested at each of the aforementioned time points (15 min, 6 h, and 24 h). The roots were separated from the soil by gentle shaking, washed with 0.01 M CaCl2 for 1 min, and washed again with distilled water to remove any radiotracers on the root surfaces. The roots and shoots were separately harvested, freeze-dried (Labconco FreeZone Freeze-Dry System, Kansas City, MO, USA), and pulverised in a ball mill (MM301; Retsch GmbH, Haan, Germany). The 14C-labelled plant tissues were ashed in an OX400 biological oxidiser (Harvey Instruments Corp., Hillsdale, NJ, USA). Free 14CO2 was captured with Oxosol scintillant (National Diagnostics, Atlanta, GA, USA), and the 14C activity was measured by liquid scintillometry (Wallace EG&G, Milton Keynes, UK). The 35S was extracted from 200 μg plant tissue with 1.5 mL SOLUENE-350 (PerkinElmer, Waltham, MA, USA) for 24 h and centrifuged at 5000×g and 20 °C for 5 min. Then 0.4-mL extracts were mixed with 4 mL ScintiSafe 3 scintillation cocktail (Fisher Scientific, Loughborough, UK), and 35S activity was detected with a Wallace 1404 liquid scintillation counter (Wallace EG&G, Milton Keynes, UK) (Jones et al. 2018a). C and N content and 13C and 15N incorporation in wheat and oilseed rape were determined with an elemental analysis-stable isotope mass spectrometer (IsoPrime100; Isoprime Ltd., Cheadle Hulme, UK).
The rhizosphere soil in a single rhizotube was divided into four 3 g portions. One portion was extracted with 15 mL of 0.01 M CaCl2 (35S-labelled) or 15 mL of 1 M KCl (14C-labelled) to detect free labelled cysteine and produced 35SO42−. The second was fumigated with 1 mL alcohol-free CHCl3 for 24 h, and residual CHCl3 was removed by vacuuming 5 five times for 50 min each time. The soil sample was extracted with 15 mL of 0.01 M CaCl2 or 15 mL of 1 M KCl to detect 14C and 35S in the microbial biomass (Vong et al. 2004). The third was extracted five times with 50 mL of 1 M KCl to measure labelled cysteine and SO42− adsorbed to the soil particles (Cao et al. 2013). The moisture content of the fourth portion was determined by oven-drying the soil at 105 °C for 24 h. The solution was added to the soil samples and shaken at 180 rpm for 1 h. The samples were then centrifuged at 6000×g and 20 °C for 15 min. Then, either 0.5 mL purified water or 0.5 mL of 1 M BaCl2 was added to 1 mL of the 0.01 M CaCl2 extracts, and the mixtures were centrifuged at 18,000×g and 20 °C for 5 min. 35S activity was detected, and the difference between samples was attributed to SO42− activity derived from labelled cysteine. The BaCl2 precipitated the SO42− to BaSO4 but had a negligible effect on the S-containing amino acids in the soil (Ma et al. 2020a, 2020b). The 14C and 35S activity levels were determined with a Wallace 1404 liquid scintillation counter (Wallace EG&G, Milton Keynes, UK) (Ma et al. 2020d). The 14C and 35S levels in the microbial biomass were determined by the fumigation-extraction method (Vong et al. 2004). The 14C was extracted with 1 M KCl, and the 35S was extracted from fumigated and unfumigated soil samples with 0.01 M CaCl2. The 14C and 35S levels were measured as previously described. The MB-C and MB-S were calculated using the conversion factor 2.22 for C (Jenkinson et al. 2004) and 2.86 for S (Vong et al. 2004).
Cysteine mineralisation in bulk soil and rhizosphere
Oilseed rape and wheat seeds were separately sown in rhizotubes containing 12.5 g agricultural brown earth soil and cultivated for 17 days. Sixteen rhizotubes were planted with oilseed rape, 16 were sown with maize, and another 16 contained only bulk soil. The soil samples in four tubes per treatment were combined into a single replicate. The water content and the environmental conditions were the same for all rhizotubes. Each 5-g soil sample was placed in a sterile 50-cm3 polypropylene container to which 0.5 mL of 50 μM 14C-Cys, 200 μM 14C-Cys, 1000 μM 14C-Cys, 50 μM 14C-Cys + 15 mg kg−1 N, or 14C-50 μM Cys + 1 mM Na2SO4 was added. The 14C activity was 3.51 kBq mL−1, N was supplied in the form of NH4NO3-N, and the NH4NO3 and NaSO4 were dissolved in the cysteine solution. The 14CO2 evolved from the soil was captured in a phial containing a 1 M NaOH trap and incubated at 20 °C. The 14CO2 traps were replaced at 1, 3, 6, 9, 24, 48, and 96 h, and their 14CO2 levels were determined by liquid scintillometry as previously described. To account for the 14CO2 produced by the intrinsic microbial community in the 14C-labelled plant material (phyllosphere), control incubations were performed in the absence of soil (Jones et al. 2018b).
Calculations and statistical analysis
The amounts of 13C and 15N derived from labelled cysteine and absorbed by wheat and oilseed rape were calculated by subtracting the quantities of 13C and 15N in the ‘blank’ seedlings from those in the treated seedlings (Sauheitl et al. 2009):
where Cuptake is the quantity of absorbed 13C that originated in labelled cysteine, CTotal-C is the total plant carbon, As is the % of 13C atoms in 13C/15N-cysteine-treated wheat, and Ac is the % of 13C atoms in the ‘blank’ seedlings. The equation for 15N uptake was similar to that used to calculate 13C uptake. The ratio of plant 13C and 15N uptake was calculated by dividing the total 13C addition by the total 15N addition.
The ratio of 14C uptake () by wheat and oilseed rape from labelled cysteine was calculated by subtracting the amount of 14C in the ‘blank’ seedlings from that in the treated seedlings:
where As is the 14C activity in the 14C-Cys treated plants, Ac is the 14C activity in the ‘blank’ seedlings, and 14CTotal is the total 14C activity added to the soil. The equation used to calculate 35S was similar to that used to determine 14C.
The 35S uptake after mineralisation (35Suptake ratio-min) was calculated as the 35S uptake ratio (35Suptake ratio) minus the 14C uptake ratio (intact cysteine uptake). This calculation was applied to each treatment as there were only five replicates. The linear relationship between 14C and 35S (14C = a35S + b) was shown and plotted as previously described by Ma et al. (2018).
The soils contained some cysteine and SO42− in addition to the added labelled substrates. The amounts of N and S absorbed were expressed in μM and derived from the quantity initially present in the original soil; the amounts added and calculated as follows:
where Suptake is the amount of absorbed S derived from cysteine or SO42−, Contentsoil is the amount (μM) of soil-soluble cysteine or SO42− in a single pot (12.5 g soil; Table S1), and 0.06 is the amount of added cysteine or SO42− (0.06 μM).
The contributions of S (% of total S uptake) from intact or mineralised cysteine and SO42− were calculated as follows:
where Suptake-Cys is the amount of absorbed S derived from cysteine (intact and inorganic S after mineralisation) and Suptake-SO42− is the amount of absorbed S derived from SO42−.
Amino acid mineralisation was generally biphasic. Hence, it was described by a two-step, double first-order kinetic decay model (Glanville et al. 2016; Ma et al. 2020c):
where f is the 14C remaining in the soil, a1 and a2 are the quantities of 14C partitioned between faster primary mineralisation (C pool1; primary mineralisation; microbial respiration) and slower secondary mineralisation (C pool2, biomass production) (Glanville et al. 2016), k1 and k2 are the exponential coefficients for pool1 and pool2, respectively, and t is time.
The half-life (t½) of pool1 or pool2 was calculated as follows:
The microbial C use efficiency (CUE) of the 14C-labelled substrates was calculated as follows (Glanville et al. 2016; Jones et al. 2018b):
All data are presented as means ± SE. A Shapiro-Wilk test was used to assess normality before applying one-way ANOVA followed by Tukey’s post hoc test (P < 0.05) to identify significant differences among treatments. The exponential decay equation was fitted to the experimental data in SigmaPlot v. 10.0 (SPSS Inc., Chicago, IL, USA). Graphs were plotted with Origin v. 8.1 (OriginLab Corp., Northampton, MA, USA).
Results
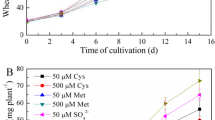
The 14C and 13C assays showed that the plants absorbed 0.1–0.9% of the total added cysteine (Fig. 2). After 15 min, the wheat and oilseed rape absorbed 0.18 and 0.10% of the total 14C-cysteine, respectively. 13C labelling indicated that they absorbed 0.15 and 0.38% of the cysteine, respectively. At 6 h, the 14C and 13C uptake levels nearly reached their maxima (wheat, 14C: 0.37 and 13C: 0.35; oilseed rape, 14C: 0.79 and 13C: 0.81). However, no further significant change in these values was observed at 24 h. In contrast, the 15N and 35S uptake rates markedly increased with time. At 24 h, the wheat and oilseed rape uptake levels ranged from 1.6 to 11.5% of the total added 35S and 12.3 to 7.6% of the 15N. The oilseed rape and wheat absorbed similar amounts of 15N at 24 h, while the oilseed rape absorbed ~6-fold more 35S than did the wheat.
The 14C and 13C assays indicated that N addition did not affect intact cysteine uptake in the plants. Nevertheless, N addition increased wheat 35S and 15N uptake and decreased oilseed 35S and 15N uptake. Oilseed rape had a smaller biomass than did wheat but higher uptake ratios of intact cysteine and S derived from cysteine (Fig. 2). After 14C was absorbed, 11.1, 31.3, and 54.1% of the total uptake were released as 14CO2 from the wheat leaves, while 45.6, 29.5, and 69.3% of the total uptake were released as 14CO2 from the oilseed rape leaves at 15 min, 6 h, and 24 h, respectively.
A linear relationship existed between 14C and 35S (14C = a35S + b) for wheat and oilseed rape. Here, ‘a’ represents the ratio of intact cysteine uptake to the total 35S uptake. When a = 1, all absorbed 14C is in the organic molecular form. The intact cysteine uptake ratios accounting for the total S uptake and based on 14C and 35S regression were 76.3, 64.3, and 22.8% for wheat and 82.3, 23.1, and 8.0% for oilseed rape at 15 min, 6 h, and 24 h, respectively. According to the 13C and 15N regression, however, the ratios were 14.2, 6.1, and 5.2% for wheat and 56.4, 17.3, and 11.2% for oilseed rape at 15 min, 6 h, and 24 h, respectively (Fig. 2).
After accounting for the soil cysteine and SO42− content, we established that wheat and oilseed rape use SO42− as their main S source. The uptake of inorganic S derived from cysteine increased over time. For wheat, the uptake of S from cysteine was higher than that from SO42− at 15 min. In contrast, oilseed rape utilised relatively more SO42− than cysteine after 15 min. N addition significantly enhanced cysteine-S utilisation by wheat but diminished its utilisation by oilseed rape after 24 h. S uptake from cysteine and SO42− was ~10-fold higher for oilseed rape than for wheat (Fig. 3).
Uptake of S from cysteine (Cys) and sulphate by wheat (A) and oilseed rape (B) derived from added and native cysteine/SO42− in soil and calculated from 14C and 35S labelling at 15 min, 6 h, and 24 h. A portion of the inorganic S derived from cysteine mineralisation was included. Data are means ± SE of five replicates
Intact cysteine-S uptake accounted for 46.1% of the total S uptake by wheat but only 9.8% of the total S uptake by oilseed rape at 15 min. The contribution of intact cysteine-S declined to 5.6 and 1.1% for wheat and oilseed rape, respectively, after 24 h. At the same time, the contributions of intact cysteine and inorganic S derived from cysteine accounted for 24.5 and 13.6% of the total S uptake by wheat and oilseed rape, respectively. However, the contribution of SO42− accounted for 75.5 and 86.4% of the total S uptake by wheat and oilseed rape, respectively (Fig. 4).
Contributions of S from organic amino acids and inorganic S derived from added and native Cys/SO42− in soil by wheat and oilseed rape as calculated from 14C and 35S labelling at 15 min, 6 h, and 24 h. Data are means ± SE of five replicates. Cys, cysteine; IS-Cys, inorganic sulphur derived from cysteine
Fifteen minutes after cysteine addition, only 11.8–15.2% of the added cysteine remained in the soil solution, whereas 56.2–55.9% of the 14C and 14.3–11.7% of the 35S were retained in the microbial biomass (Fig. 5). After 24 h, the 14C in the microbial biomass declined to 14.3–17.1%, while the 35S in the microbial biomass rose to 19.0–28.8%. After 24 h, the amount of 14CO2 released from the soil increased to 49.0–66.2%, whereas 52.7–54.5% of the total 35S-cysteine was released as SO42−. At 15 min, 12.6–20.0% of the 14C-cysteine was adsorbed to soil particles, and only a trace amount was detected after 24 h. Almost half the added SO42− was retained in the soil solution, and 45.2–41.3% of it was retained in the microbial biomass by 24 h. N addition significantly increased the ratio of 14C retained in the microbial biomass but had no effect on 35S-MB and decreased the 14CO2 release ratio.
The 14C-cysteine decomposed rapidly in the soil (t1/2 = 1.37 h). This rate did not substantially differ between the wheat and oilseed rape rhizospheres or the bulk soil (Fig. 6). N and S addition had negligible influences on the cysteine decomposition rate. However, soil cysteine decomposition decreased with increasing soil cysteine concentration. The t1/2 were 2.79–2.85 h and 6.53–6.61 h for 200 μM cysteine and 1000 μM cysteine, respectively. Cysteine concentration and N and S addition had no apparent impact on C use efficiency (Table S2).
Discussion
Plant cysteine uptake from soil
In the rhizosphere, the roots compete with soil microorganisms for OS and ON. Microbes consume most soil organic matter, and plant roots can only access limited OS and ON (Hill et al. 2013; Kuzyakov and Xu 2013; Näsholm et al. 2009). In planted soils, amino acid turnover usually occurs within < 1 h (Farrell et al. 2014; Jones et al. 2009). Here, t1/2 was 1.37 h for cysteine. The ability of soil microorganisms to decompose OS determines the amount of intact OS available to roots. Fifteen minutes after cysteine addition to the rhizosphere, only 11.8–15.2% of the 14C remained in the soil solution, whereas 55.9–56.2% was retained in the microbial biomass, and 6.2–8.7% was mineralised to 14CO2 by soil microorganisms. In contrast, the roots acquired only 0.10–0.18% of the total 14C over the same time. The observed increases in 13C and 14C indicated that oilseed rape and wheat absorbed intact soil cysteine. When cysteine was mineralised, its C was transformed to CO2, and plant roots could only access N and S. Plant cysteine uptake reached a maximum at 6 h. By 24 h, the soil cysteine had been depleted. This finding was consistent with the fact that negligible cysteine remained in the soil solution. However, most of the labelled cysteine-derived nutrients were utilised by the plants only after mineralisation to 15NH4+, 15NO3−, and 35SO42−. Microorganisms outcompete plant roots for free amino acids in the soil solution. In the rhizosphere, microbial activity may be an order of magnitude higher than it is in bulk soil (Owen and Jones 2001). Hence, the roots had access to less cysteine than did the microorganisms (Ganeteg et al. 2017; Kuzyakov and Xu 2013; Ma et al. 2018).
Inorganic S and N liberated during microbial amino acid mineralisation are available as plant nutrients (Seegmüller and Rennenberg 2002). Here, cysteine rapidly decomposed to sulphate; 30.2–36.7% of the 35S cysteine was converted to SO42− after 15 min, and the roots utilised the inorganic N and S. The microbial biomass absorbed the cysteine within 15 min, whereas plant uptake of cysteine-derived nutrients was far slower. The latter process depended on the rates of microbial inorganic N and S liberation. After 24 h, wheat and oilseed rape absorbed 1.6–11.5% of 35S and 7.6–12.3% of 15N, respectively. Over the long term, then, plants prevail in terms of N and S acquisition as soil-to-root nutrient flow is unidirectional (Kuzyakov and Xu 2013). Despite strong competition for nutrients between plants and rhizosphere microorganisms, temporal niche differentiation induces mutualistic relationships that prevent N and S losses resulting from leaching during periods of minimal or no root uptake. Thus, roots receive a steady supply of available soil N and S (Kuzyakov and Xu 2013).
The rhizosphere experiments in this study demonstrated that wheat and oilseed rape differed in terms of their ability to utilise OS. Wheat roots used more intact cysteine than SO42−, while oilseed rape absorbed 3-fold more S from SO42− than from cysteine after 15 min. Thus, oilseed rape might utilise SO42− as its main S source. In the soil, SO42− predominates in the highly bioavailable S pool. Moreover, oilseed rape efficiently utilises SO42− to meet its high S demand. Plants with coarse root systems (with various root exudates) such as oilseed rape harbour homogeneous microbial communities specialising in S immobilisation/remobilisation (Vong et al. 2002). Here, however, wheat and oilseed rape had minimal impact on root microbial OS decomposition. Hence, the differences between wheat and oilseed rape in terms of their capacity to utilise various S sources are determined by their individual root uptake characteristics rather than by microbial competition in the rhizosphere.
Approximately 46–72% of the 14C-cysteine absorbed by wheat roots was released from the leaves in the form of CO2. In contrast, only 11–54% of the 14C-cysteine was released as CO2 from oilseed rape leaves after 24 h. Hence, wheat has lower cysteine C-use efficiency than does oilseed rape, and the latter has a higher S demand than does the former (Scherer 2001). Wheat can immobilise more OS than oilseed rape, which might be due to its higher root biomass. Several studies used 13C/15N dual-labelled organic N to measure the extent to which organic N meets plant N requirements (Ganeteg et al. 2017; Kuzyakov and Xu 2013; Ma et al. 2018). However, these studies did not consider foliar 13C release and may have underestimated the actual organic N contribution. Long-term OS uptake from the soil may have been underestimated because no other S-containing compounds such as glutathione and methionine were evaluated. Furthermore, cysteine was applied to the soil only by pulse addition and not continuously. In this study, the actual contribution might have been overestimated because we used a high cysteine concentration (Näsholm et al. 2009). Cysteine may undergo numerous changes in the soil, and a fraction of the 14C/13C uptake from cysteine may be in the form of oxidation products such as cystine rather than intact cysteine per se.
Soil cysteine-derived S, N, and C cycling
Elucidation of the mechanism by which microorganisms decompose organic S is crucial for the regulation and prediction of soil S cycling. Cysteine decomposition by soil microorganisms involves rapid amino acid immobilisation into microbial biomass, the release of sulphate, and the reutilisation of the sulphate by soil microorganisms (Ma et al. 2020a, 2020b). Here, only 11.8–15.2% of the cysteine remained in the soil solution after 15 min. In contrast, 56.2–55.9% of the 14C and 14.3–11.7% of the 35S were retained in the microbial biomass, and 30.2–36.7% of the SO42− was released. Thus, the first and second steps (immobilisation into microbial biomass and SO42− release, respectively) may occur within minutes. In another study, 70% of the S originating from cysteine was retained in microbial biomass within 2 min (Ma et al. 2021a).
Rapid microbial S immobilisation predominates in S flux after cysteine is added to the soil. Individual amino acids may be removed from the soil solution (Czaban et al. 2016; Hill and Jones 2019) and mineralised to CO2 and SO42− (Hill and Jones 2019; Wilkinson et al. 2014) within minutes to hours. The amino acids may serve as important N and S sources even at relatively low soil concentrations. The thiol group of cysteine is readily oxidised and is the key moiety in microbial and plant S cycling (Romero et al. 2014). Microorganisms may have mineralised the S without incorporating it, but relatively high C levels were retained in the microbial biomass. Furthermore, the microorganisms retained more of the C than the S originating from cysteine. Microbial OS mineralisation induced by microbial C demand could add to the existing plant S supply. After heterotrophs absorb the substrate, the N:C and S:C ratios may exceed certain thresholds leading to net N and S mineralisation, and extra N and S can be released as SO42− and NH4+ to maintain microbial biomass stoichiometry (Fan et al. 2020; Manzoni et al. 2017; Mooshammer et al. 2014; Wei et al. 2020). At 6 h and 24 h, 35S MB increased, and the sulphate released was reutilised by soil microorganisms. Moreover, 12.6–20.0% of the 14C-cysteine was adsorbed to soil particles after 15 min. Part of this fraction might have been absorbed by the roots as the 14C content had increased after 6 h (Cao et al. 2013). In addition, anaerobic processes, sulphate reduction and subsequent binding to minerals (such as FeS), and stable incorporation into microbial necromass might be responsible for the unknown part of this fraction.
Factors influencing cysteine uptake
S and N assimilation are closely linked (Schneider et al. 2019). In the rhizosphere, N addition promoted wheat 15N and SO42− uptake but inhibited both processes in oilseed rape. The differences between wheat and oilseed rape in terms of N and S uptake, transport, and demand might account for this discrepancy. Here, N addition had a negligible impact on cysteine decomposition by soil microorganisms, possibly because there was adequate N supply in the agricultural soil (Ma et al. 2020b).
Plant amino acid uptake reaches a maximum at high soil concentrations (Jones et al. 2005). Local soil amino acid levels after earthworm activity and clover decomposition can be as high as 45.3 mM and 2.7 mM, respectively, which suffice for root uptake (Hill et al. 2019a). High transient soil organic N and S concentrations occur after cell death. Soil 14C-cysteine decomposition is rapid (t1/2 = 1.37 h), but this rate decreases with increasing cysteine concentration. For example, t1/2 = 6.53–6.61 h for 1 mM cysteine. Plant cysteine uptake may reach a maximum at high concentrations wherein microbial cysteine utilisation is the lowest. Hence, plant OS uptake occurs mainly in organic-rich soil patches (Hill et al. 2019a; Jones et al. 2005).
Conclusions
Wheat and oilseed rape can absorb intact cysteine from the rhizosphere even in the presence of soil microorganisms. The latter rapidly decompose cysteine, and the roots absorb mineralised N and S. Therefore, plants predominate in long-term N and S acquisition. Plant cysteine uptake reaches a maximum at high concentrations and occurs primarily in organic-rich soil patches. We conducted this research within agriculture soil, and the role of S-containing amino acids such as cysteine and methionine in plant S acquisition within natural soil requires further research.
References
Bona FDD, Monteiro FA (2010) Nitrogen and sulfur fertilization and dynamics in a Brazilian entisol under pasture. Soil Sci Soc Am J 74:1248–1258
Cao X, Chen X, Li X, Yuan L, Wu L, Zhu Y (2013) Rice uptake of soil adsorbed amino acids under sterilized environment. Soil Biol Biochem 62:13–21
Cao X, Ma Q, Zhong C, Yang X, Zhu L, Zhang J, Jin Q, Wu L (2016) Elevational variation in soil amino acid and inorganic nitrogen concentrations in Taibai Mountain, China. PLoS One 11:e157979
Ciaffi M, Paolacci AR, Celletti S, Catarcione G, Kopriva S, Astolfi S (2013) Transcriptional and physiological changes in the S assimilation pathway due to single or combined S and Fe deprivation in durum wheat (Triticum durum L.) seedlings. J Exp Bot 64:1663-1675
Czaban W, Rasmussen J, Nicolaisen M, Fomsgaard IS (2016) Dissipation kinetics of asparagine in soil measured by compound-specific analysis with metabolite tracking. Biol Fertil Soils 52:911–916
Dippold M, Biryukov M, Kuzyakov Y (2014) Sorption affects amino acid pathways in soil: implications from position-specific labeling of alanine. Soil Biol Biochem 72:180–192
Dong Y, Silbermann M, Speiser A, Forieri I, Linster E, Poschet G, Samami AA, Wanatabe M, Sticht C, Teleman AA (2017) Sulfur availability regulates plant growth via glucose-TOR signaling. Nat Commun 8:1174
Fan R, Du J, Liang A, Lou J, Li J (2020) Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry. Biol Fertil Soils 56:1109–1120
Farrell M, Macdonald LM, Hill PW, Wanniarachchi SD, Farrar J, Bardgett RD, Jones DL (2014) Amino acid dynamics across a grassland altitudinal gradient. Soil Biol Biochem 76:179–182
Ganeteg U, Ahmad I, Jämtgård S, Aguetoni-Cambui C, Inselsbacher E, Svennerstam H, Schmidt S, Näsholm T (2017) Amino acid transporter mutants of Arabidopsis provides evidence that a non-mycorrhizal plant acquires organic nitrogen from agricultural soil. Plant Cell Environ 40:413–423
Glanville HC, Hill PW, Schnepf A, Oburger E, Jones DL (2016) Combined use of empirical data and mathematical modelling to better estimate the microbial turnover of isotopically labelled carbon substrates in soil. Soil Biol Biochem 94:154–168
Hill EJ, Jones DL, Paterson E, Hill PW (2019a) Hotspots and hot moments of amino acid N in soil: real-time insights using continuous microdialysis sampling. Soil Biol Biochem 131:40–43
Hill PW, Jones DL (2019) Plant–microbe competition: does injection of isotopes of C and N into the rhizosphere effectively characterise plant use of soil N? New Phytol 221:796–806
Hill PW, Broughton R, Bougoure J, Havelange W, Newsham KK, Grant H, Murphy DV, Clode P, Ramayah S, Marsden KA (2019b) Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecol Lett 22:2111–2119
Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang 8:613–619
Hill PW, Marsden KA, Jones DL (2013) How significant to plant N nutrition is the direct consumption of soil microbes by roots? New Phytol 199:948–955
Honsel A, Kojima M, Haas R, Frank W, Sakakibara H, Herschbach C, Rennenberg H (2012) Sulphur limitation and early sulphur deficiency responses in poplar: significance of gene expression, metabolites, and plant hormones. J Exp Bot 63:1873–1893
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Jones DL, Kielland K (2012) Amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol Biochem 55:60–69
Jones DL, Kielland K, Sinclair FL, Dahlgren RA, Newsham KK, Farrar JF, Murphy DV (2009) Soil organic nitrogen mineralization across a global latitudinal gradient. Global BIogeochem Cy 23:G81016
Jones DL, Magthab EA, Gleeson DB, Hill PW, Sánchez-Rodríguez AR, Roberts P, Ge T, Murphy DV (2018a) Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol Biochem 117:72–82
Jones DL, Olivera-Ardid S, Klumpp E, Knief C, Hill PW, Lehndorff E, Bol R (2018b) Moisture activation and carbon use efficiency of soil microbial communities along an aridity gradient in the Atacama Desert. Soil Biol Biochem 117:68–71
Jones DL, Shannon D, Junvee-Fortune T, Farrar JF (2005) Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol Biochem 37:179–181
Kopittke PM, Dalal RC, Menzies NW (2016) Sulfur dynamics in sub-tropical soils of Australia as influenced by long-term cultivation. Plant Soil 402:211–219
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Liu Y, Ge T, Ye J, Liu S, Shibistova O, Wang P, Wang J, Li Y, Guggenberger G, Kuzyakov Y, Wu J (2019) Initial utilization of rhizodeposits with rice growth in paddy soils: rhizosphere and N fertilization effects. Geoderma 338:30–39
Ma Q, Cao X, Xie Y, Gu Y, Feng Y, Mi W, Yang X, Wu L (2017) Effect of pH on the uptake and metabolism of glycine in pak choi (Brassica chinensis L.). Environ Exp Bot 133:139–150
Ma Q, Wu L, Wang J, Ma J, Zheng N, Hill PW, Chadwick DR, Jones DL (2018) Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: an in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Biol Biochem 125:319–327
Ma Q, Luo Y, Wen Y, Hill PW, Chadwick DR, Wu L, Jones DL (2020a) Carbon and sulphur tracing from soil organic sulphur in plants and soil microorganisms. Soil Biol Biochem 150:107971
Ma Q, Wen Y, Pan W, Macdonald A, Hill PW, Chadwick DR, Wu L, Jones DL (2020b) Soil carbon, nitrogen, and sulphur status affects the metabolism of organic S but not its uptake by microorganisms. Soil Biol Biochem 149:107943
Ma Q, Wen Y, Wang D, Sun X, Hill PW, Macdonald A, Chadwick DR, Wu L, Jones DL (2020c) Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol Biochem 144:107760
Ma Q, Wen Y, Ma J, Macdonald A, Hill PW, Chadwick DR, Wu L, Jones DL (2020d) Long-term farmyard manure application affects soil organic phosphorus cycling: a combined metagenomic and 33P/14C labelling study. Soil Biol Biochem 149:107959
Ma Q, Kuzyakov Y, Pan W, Tang S, Chadwick DR, Wen Y, Hill PW, Macdonald A, Ge T, Si L, Wu L, Jones DL (2021a) Substrate control of sulphur utilisation and microbial stoichiometry in soil: Results of 13C, 15N, 14C, and 35S quad labelling. ISME J. https://doi.org/10.1038/s41396-021-00999-7
Ma Q, Pan W, Tang S, Sun X, Xie Y, Chadwick DR, Hill PW, Si L, Wu L, Jones DL (2021b) Maize and soybean experience fierce competition from soil microorganisms for the uptake of organic and inorganic nitrogen and sulphur: a pot test using 13C, 15N, 14C, and 35S labelling. Soil Biol Biochem 157:108260
Manzoni S, Čapek P, Mooshammer M, Lindahl BD, Richter A, Šantrůčková H (2017) Optimal metabolic regulation along resource stoichiometry gradients. Ecol Lett 20:1182–1191
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Owen AG, Jones DL (2001) Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol Biochem 33:651–657
Ozols J (1990) Amino acid analysis. Methods Enzymol 182:587–601
Paungfoolonhienne C, Lonhienne TGA, Mudge SR, Schenk PM, Christie M, Carroll BJ, Schmidt S (2010) DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth. Plant Physiol 153:799–805
Paungfoolonhienne C, Lonhienne TG, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S (2008) Plants can use protein as a nitrogen source without assistance from other organisms. P Natl Acad Sci USA 105:4524–4529
Planta J, Xiang X, Leustek T, Messing J (2017) Engineering sulfur storage in maize seed proteins without apparent yield loss. P Natl Acad Sci USA 114:11386–11391
Prodhan MA, Jost R, Watanabe M, Hoefgen R, Lambers H, Finnegan PM (2017) Tight control of sulfur assimilation: an adaptive mechanism for a plant from a severely phosphorus-impoverished habitat. New Phytol 215:1068–1079
Vong PCV, Lasserre-Joulin F, Guckert A (2002) Mobilization of labelled organic sulfur in rhizosphere of rape and barley and in non-rhizosphere soil. J Plant Nutr 25:2191–2204
Romero LC, Aroca MN, Laureano-Marín AM, Moreno I, García I, Gotor C (2014) Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant 7:264–276
Sauheitl L, Glaser B, Weigelt A (2009) Uptake of intact amino acids by plants depends on soil amino acid concentrations. Environ Exp Bot 66:145–152
Scherer HW (2001) Sulphur in crop production — invited paper. Eur J Agron 14:81–111
Schneider S, Schintlmeister A, Becana M, Wagner M, Woebken D, Wienkoop S (2019) Sulfate is transported at significant rates through the symbiosome membrane and is crucial for nitrogenase biosynthesis. Plant Cell Environ 42:1180–1189
Seegmüller S, Rennenberg H (2002) Transport of organic sulfur and nitrogen in the roots of young mycorrhizal pedunculate oak trees (Quercus robur L.). Plant Soil 242:291–297
Vong PC, Dedourge O, Guckert A (2004) Immobilization and mobilization of labelled sulphur in relation to soil arylsulphatase activity in rhizosphere soil of field-grown rape, barley and fallow. Plant Soil 258:227–239
Warren CR (2013) Quaternary ammonium compounds can be abundant in some soils and are taken up as intact molecules by plants. New Phytol 198:476–485
Wei X, Razavi BS, Hu Y, Xu X, Zhu Z, Liu Y, Kuzyakov Y, Li Y, Wu J, Ge T (2019) C/P stoichiometry of dying rice root defines the spatial distribution and dynamics of enzyme activities in root-detritusphere. Biol Fertil Soils 55:251–263
Wei X, Zhu Z, Liu Y, Luo Y, Deng Y, Xu X, Liu S, Richter A, Shibistova O, Guggenberger G, Wu J, Ge T (2020) C:N:P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol Fertil Soils 56:1093–1107
Wilkinson A, Hill PW, Farrar JF, Jones DL, Bardgett RD (2014) Rapid microbial uptake and mineralization of amino acids and peptides along a grassland productivity gradient. Soil Biol Biochem 72:75–83
Wyngaard N, Cabrera ML (2015) Measuring and estimating sulfur mineralization potential in soils amended with poultry litter or inorganic fertilizer. Biol Fertil Soils 51:545–552
Funding
This work was supported by the the National Key Research and Development Program of China (2020YFD1100402), the National Natural Science Foundation of China (31872180, 31572194), and UK-China Virtual Joint Centre for Agricultural Nitrogen (CINAg, BB/N013468/1), which is jointly supported by the Newton Fund, via UK BBSRC and NERC, and the Chinese Ministry of Science and Technology.
Author information
Authors and Affiliations
Contributions
QXM and DLJ designed research, conduct the experiments, and write the manuscript; PWH assisted in soil sampling, and PWH, DRC, and LHW revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 45 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Q., Hill, P.W., Chadwick, D.R. et al. Competition for S-containing amino acids between rhizosphere microorganisms and plant roots: the role of cysteine in plant S acquisition. Biol Fertil Soils 57, 825–836 (2021). https://doi.org/10.1007/s00374-021-01572-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01572-2