Abstract
Introduction
HTL0018318 is a selective muscarinic M1 receptor partial agonist under development for the symptomatic treatment of dementias, including Alzheimer’s disease. Clinically, HTL0018318 would likely be used alone or in conjunction with cholinesterase inhibitors (e.g. donepezil).
Objective
We investigated the safety, tolerability, and pharmacokinetics of HTL0018318 given alone and in combination with donepezil.
Methods
This was a randomized, double-blind, placebo-controlled trial in 42 (to deliver 36 with combination treatment) healthy elderly subjects investigating the effects of oral HTL0018318 15 and 25 mg given alone and combined with donepezil 10 mg at steady state on adverse events (AEs), vital signs, saliva production, sleep quality, pulmonary function, subjective feelings, and pharmacokinetics.
Results
AEs were reported by lower percentages of subjects after HTL0018318 alone than after donepezil alone. There was no increase in the percentage of subjects reporting AEs after co-administration than after donepezil alone. Supine systolic blood pressure was 1.6 mmHg (95% confidence interval [CI] −3.1 to −0.1) lower after HTL0018318 alone than after combination treatment. This was comparable with results from placebo alone: 1.7 mmHg (95% CI −3.2 to 0.2) lower than with combination treatment. Supine pulse rate was 3.3 bpm (95% CI 1.5–5.1) higher after HTL0018318 alone than with co-administration. HTL0018318 and donepezil did not meaningfully affect each other’s pharmacokinetics.
Conclusion
HTL0018318 was well tolerated when given alone and in combination with donepezil. HTL0018318 and donepezil do not demonstrate pharmacokinetic or pharmacodynamic interactions, indicating that HTL0018318 can be safely administered in combination with donepezil.
Clinical trial registration
Netherlands Trial register identifier NL5915, registered on 28 October 2016.
Similar content being viewed by others
M1 muscarinic receptor partial agonist HTL0018318, both alone and in combination with donepezil, was well tolerated in elderly subjects. |
HTL0018318 and donepezil did not affect each other’s pharmacokinetics. |
1 Introduction
Alzheimer’s disease (AD) is characterised by a significant and progressive loss of acetylcholine-producing neurons in the brain [1], which is correlated with the degree of cognitive decline [2, 3]. The current standard of care consists of cholinesterase inhibitors, such as donepezil, that reduce the breakdown of synaptic acetylcholine and consequently enhance cholinergic transmission in the brain. The efficacy of cholinesterase inhibitors is modest, and dosing is limited by side effects caused by non-selective enhancement of cholinergic transmission at other acetylcholine receptor subtypes located throughout the body [4, 5]. Another approach to improve cholinergic function in AD might be agonism or modulation of the M1 subtype of the muscarinic acetylcholine receptors (M1 mAChR). The M1 mAChR is the predominant subtype in the central nervous system and is expressed in areas of the brain associated with cognitive processes, such as the prefrontal cortex, neostriatum, and hippocampus [6, 7]. In patients with AD, the M1 mAChR is relatively well preserved [8]. Previously, muscarinic receptor agonists have been taken into development; the M1/M4 agonist xanomeline and the M1 allosteric bitopic agonist GSK1034702 showed promising early clinical effects on cognitive function [9, 10]; however, further development of both compounds was terminated because of side effects caused by binding of the compounds to muscarinic receptors outside of the central nervous system.
HTL0018318 is a novel selective M1 mAChR partial agonist. Pre-clinical data show that HTL0018318 has approximately a twofold selectivity for M1 over M4 receptors with no detectable functional agonist activity at human M2 and M3 receptors [11]. Multiple doses in healthy elderly humans resulted in an acceptable side effect profile, with hyperhidrosis, nausea, and hot flushes the most prevalent adverse events (AEs) [12]. As a treatment for AD, HTL0018318 will very likely be given in combination with standard-of-care cholinesterase inhibitors such as donepezil. As both HTL0018318 and cholinesterase inhibitors increase cholinergic activity, the aim of this study was to investigate whether HTL0018318 can be safely administered in combination with donepezil. Although HTL0018318 was found not to interact with cytochrome P450s (CYPs) or drug transporters, and donepezil is reported to be only a weak inhibitor of CYP2D6 or CYP3A4 (half maximal inhibitory concentration 50–130 µM [13]), suggesting a low probability for drug–drug interactions, this study evaluated the effects on pharmacokinetic characteristics of both HTL0018318 and donepezil when at steady state.
2 Methods
2.1 Trial Design and Subjects
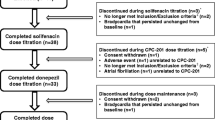
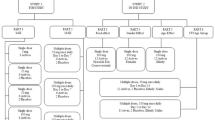
This was a randomized, fixed-sequence, double-blind, placebo-controlled trial investigating multiple doses of HTL0018318 at 15 mg (12 subjects; eight active and four placebo) and 25 mg (24 subjects; 16 active and eight placebo) given with and without donepezil at steady state in healthy elderly subjects. Open-label donepezil (taken in the evening) was up-titrated to steady-state plasma concentrations by administering donepezil 5 mg once daily for 5 consecutive days followed by donepezil 10 mg once daily (therapeutic dose level) for 15 consecutive days. Subsequently, the donepezil treatment was combined with HTL0018318 or placebo dosed daily for 5 consecutive days (taken in the morning). This was followed by a washout period of 20 days and subsequent administration of HTL0018318 or placebo alone, daily for 5 consecutive days, at the same dose as previously administered in combination (Fig. 1). As it was expected that some subjects would withdraw from study participation because of side effects of donepezil during the donepezil run-in period, this treatment sequence prevented unnecessary exposure to HTL0018318 in subjects who had not previously completed the donepezil run-in phase. During the two periods in which HTL0018318 or placebo were administered, safety and pharmacokinetic measurements were performed daily. The study was run in three cohorts to allow within-study modification of the dose of HTL0018318 in the event that an unexpected interaction occurred. According to protocol, the first cohort was administered HTL0018318 at 15 mg and the second cohort received HTL0018318 at 25 mg. The dose level of the third cohort (25 mg) was based on blinded safety and pharmacokinetic data from the first and second cohorts.
Elderly subjects aged 65–80 years (inclusive) participated in the study. Subjects were eligible if they were in good health, with a maximum resting blood pressure of up to 150/90 mmHg and a heart rate between 45 and 100 bpm at screening. The main exclusion criteria were current or past history of any illness interfering with the study objectives, the use of antihypertensive drugs and products that influence CYP3A4 or CYP2D6 and clinically relevant abnormalities on a 24-h Holter electrocardiogram (ECG).
2.2 Materials
HTL0018318 was administered orally as an aqueous solution in 100 mL. Water was used as placebo. To mask the difference in taste between HTL0018318 and placebo, a peppermint strip (Listerine) was administered 1 minute before and after the administration of the oral solution. In humans, the time to maximum observed plasma concentration (tmax) of HTL0018318 was 1–2 h, and the half-life was approximately 16 h, which permits once-daily dosing [12, 14]. Steady state was reached after two or three daily doses [12]. Donepezil (manufactured by Aliud Pharma GmbH, Laichingen, Germany) was administered as 5 mg tablets. Donepezil has a tmax of 3–4 h and a half-life of approximately 70 h [13].
2.3 Safety and Tolerability Assessments
A detailed overview of the timing of all measurements is provided in Table S1 in the electronic supplementary material (ESM). AEs were summarised per treatment (i.e. HTL0018318 15 or 25 mg or placebo) and per study phase (i.e. donepezil alone, HTL0018318/placebo + donepezil, and HTL0018318/placebo alone). The AEs that were reported when donepezil was administered alone were summarised per treatment given after this phase (e.g. AEs reported when donepezil was administered alone by subjects who were to receive HTL0018318 15 mg later on during the study). A subset of AEs with a possible relation to increased cholinergic stimulation was created: hyperhidrosis, salivary hypersecretion, hypertension, tachycardia, bradycardia, nausea, diarrhoea, vomiting, constipation, insomnia, dizziness, muscle spasms, hot flush, and cold sweat.
Data were obtained for systolic and diastolic blood pressure and pulse rate, all measured in supine and standing position, safety laboratory, ECG, and 24-h Holter ECG.
Saliva production was assessed by measuring the change in weight of three Salivette® dental rolls placed in the oral cavity for 3 min. Pulmonary function was measured using the Spirostik (Accuramed), a computer-based open spirometry system. Subjective feelings were assessed using the visual analogue scale (VAS) according to Bond and Lader [15] and a VAS for nausea (0–100 mm). The Leeds Sleep Evaluation Questionnaire (LSEQ) was used to monitor changes in ease of initiating sleep, quality of sleep, ease of waking, and behaviour following wakefulness [16].
2.4 Pharmacokinetic Assessments
Plasma concentrations of donepezil and plasma and urine concentrations of HTL0018318 were determined using validated bioanalytical methods involving protein precipitation and liquid chromatography coupled with tandem mass spectrometry. The analytical range of the assay was 0.1–100 ng/mL donepezil or 0.5–1000 ng/mL HTL0018318. To determine plasma donepezil concentrations, blood samples were collected after the fifth administration of donepezil 5 mg and during the donepezil administrations at therapeutic dose level as shown in Fig. 2. The time point 15 h post donepezil dose corresponded with the time point immediately prior to HTL0018318 dosing.
To determine plasma HTL0018318 concentrations, blood samples were frequently taken on days when the first and fifth dose of HTL0018318 ± donepezil was administered. On the days between, only pre-dose pharmacokinetic samples were taken. The last pharmacokinetic blood sample was taken between 7 and 14 days after the last HTL0018318 dose (Table S1 in the ESM).
To estimate HTL0018318 urine concentrations, all urine was collected within 24 h after the first dose and within 72 h after the last dose of HTL0018318 ± donepezil.
Pharmacokinetic parameters included in the analysis were the maximum observed plasma concentration (Cmax); tmax; plasma concentration 24 h post-dose (Cmin); area under the plasma concentration–time curve (AUC) from zero to 24 h post-dose (AUC0–24), from zero to the end of the dose interval (AUC0–tau), and from zero to infinity (AUC0–inf); apparent elimination half-life (t½); apparent oral clearance (CL/F); renal clearance (CLr) and percentage of dose excreted renally as unchanged drug (Ae%); and coefficient of variation (%CV). All pharmacokinetic analyses were performed in Phoenix 64 build 6.4.0.768 using WinNonlin 6.4.
2.5 Statistical Analysis
A sample size typical of drug–drug interaction studies was chosen [17,18,19]; the study was not statistically powered. The safety and tolerability assessments of saliva measurement, pulmonary function test, VAS Bond and Lader, VAS nausea, LSEQ, and vital signs measured during the periods that HTL0018318 or placebo were administered in combination with and without donepezil were subjected to exploratory analysis. To this end, a mixed-model analysis of variance was used with treatment, period, time, treatment by period, period by time, treatment by time, and treatment by period by time as fixed factors. Subject, subject by period, and subject by time were random factors, and the pre-HTL0018318 baseline measurement per period was a covariate. In these analysis models, all means were estimated (least square means). Statistical analysis was conducted with SAS 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). The following contrasts were calculated: HTL0018318 alone versus placebo alone, HTL0018318 + donepezil versus placebo + donepezil, and HTL0018318 + donepezil versus HTL0018318 alone. Analyses were performed for HTL0018318 15 and 25 mg dose levels separately.
The effect of HTL0018318 on the pharmacokinetics of donepezil was analysed by comparing the plasma donepezil concentrations sampled pre-dose, 4 h, and 15 h after the 20th donepezil dose (i.e. prior to HTL0018318 or placebo) with the plasma donepezil concentrations at the same times of the 21st and 24th donepezil doses. The 21st and 24th donepezil doses were administered after the first and fourth HTL0018318 administrations, respectively.
The effects of donepezil on the pharmacokinetics of HTL0018318 were assessed by comparing the HTL0018318 Cmax, tmax, and AUC0–24 after the first dose of HTL0018318 + donepezil with the same parameters when HTL0018318 was administered without donepezil. Also, the HTL0018318 Cmax, AUC0–tau, tmax, and Cmin after the last dose of HTL0018318 + donepezil was compared with the same parameters when HTL0018318 was administered without donepezil. For these calculations, data for HTL0018318 15 and 25 mg were grouped together.
The ratio of each above-mentioned parameter with and without donepezil co-dosing was calculated, and the 90% confidence interval (CI) of the geometric mean was assessed.
The degree of accumulation of exposure to HTL0018318 over the study period was assessed by calculating the ratio of AUC0-tau following repeat dosing to the AUC0–tau following the first dose. To assess the effect of donepezil co-administration on accumulation, these ratios calculated during the treatment period with co-administration of donepezil and without co-administration of donepezil were compared.
Statistical analysis was performed in R version 3.3.1.
3 Results
3.1 Subjects
In total, 42 subjects enrolled in this study, of whom three withdrew because of side effects from donepezil and three were withdrawn upon re-evaluation of eligibility, all prior to co-administration of HTL0018318. The remaining 36 subjects were randomized to placebo (n = 12), HTL0018318 15 mg (n = 8), or HTL0018318 25 mg (n = 16) (Table 1).
After the first dose of HTL0018318/placebo in combination with donepezil, five subjects withdrew because of a presumably viral gastroenteritis (n = 2 on placebo, n = 3 on HTL0018318 25 mg) and one subject missed the fifth placebo dose because of this presumably viral gastroenteritis. Another two subjects were withdrawn because of non-study drug-related abnormal laboratory results after the washout period prior to first administration of HTL0018318/placebo without donepezil. In total, 28 subjects completed the study.
3.2 Safety and Tolerability
No clinically significant changes related to treatment were seen in any of the laboratory tests, ECG assessments, or 24-h Holter ECG results.
There were no significant changes in standing systolic blood pressure, supine and standing diastolic blood pressure, standing–supine blood pressure, or standing pulse rate after HTL0018318 + donepezil compared with HTL0018318 alone. Only effects on supine systolic blood pressure, supine pulse rate, and standing–supine pulse rate were observed.
Supine systolic blood pressure was significantly lower after administration of HTL0018318 25 mg without donepezil (118 mmHg), but not after administration of the 15 mg dose, compared with HTL0018318 25 and 15 mg, respectively, + donepezil (120 mmHg, mean difference of 1.6 mmHg, 95% CI −3.1 to −0.1; p=0.0378). After placebo without donepezil (118 mmHg), the supine systolic blood pressure was significantly lower than with placebo in combination with donepezil (120 mmHg, mean difference of 1.7 mmHg, 95% CI −3.2 to −0.2; p=0.0242). Administration of HTL0018318 (both 15 and 25 mg) showed no significant effects on supine systolic blood pressure when compared with placebo either in combination with or without donepezil.
Supine pulse rate was significantly lower after administration of HTL0018318 15 and 25 mg + donepezil compared with HTL0018318 alone (HTL0018318 15 mg + donepezil [64 bpm] vs. HTL0018318 15 mg without donepezil [67 bpm]: mean difference of 3.3 bpm, 95% CI 1.5–5.1; p=0.0009; HTL0018318 25 mg + donepezil [64 bpm] vs. HTL0018318 25 mg without donepezil [66 bpm]: mean difference of 1.5 bpm, 95% CI 0.2–2.9; p=0.0302). Administration of HTL0018318, both 15 and 25 mg, showed no significant effects on supine pulse rate when compared with placebo either in combination with or without donepezil.
The change in pulse rate when standing from the pulse rate when supine (delta pulse rate) was significantly lower after administration of HTL0018318 25 mg without donepezil (change of 10 bpm) than with HTL0018318 25 mg + donepezil (change of 12 bpm, mean difference of −1.6 bpm, 95% CI −3.0 to −0.2; p = 0.0252). There were no significant changes in delta pulse rate after administration of HTL0018318 15 mg or placebo without donepezil compared with treatment in combination with donepezil. The delta pulse rate after HTL0018318 25 mg without donepezil (change of 12 bpm) and in combination with donepezil (change of 10 bpm) was significantly lower than for placebo without donepezil (change of 14 bpm, mean difference of −3.7 bpm, 95% CI −6.6 to −0.8; p = 0.0137) and for placebo + donepezil (change of 15 bpm, mean difference of −3.4 bpm, 95% CI −6.2 to −0.6; p = 0.0184).
Statistically significant changes were observed in saliva production, pulmonary function forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC), LSEQ domain Quality of Sleep, and LSEQ Awake following sleep (Table S2 in the ESM). All these changes were small and not considered clinically relevant.
There were no statistically significant effects on VAS alertness, calmness, mood, and nausea after HTL0018318 + donepezil compared with HTL0018318 without donepezil.
All AEs were mild or moderate in intensity, and there were no serious AEs. The number of AEs reported when donepezil was administered alone did not increase after co-administering HTL0018318 15 mg and 25 mg. The percentages of subjects that reported AEs are shown in Table 2. Compared with HTL0018318 15 mg alone, co-administration of donepezil did increase the percentage of subjects reporting AEs. When HTL0018318 25 mg was administered, a similar percentage of subjects reported AEs in the presence and absence of donepezil. The same pattern was observed in relation to percentages of subjects that reported AEs with a (possible) relation to increased cholinergic stimulation (Table 3). The most frequently reported AEs were hot flushes, hyperhidrosis, nausea, vomiting, headache, and somnolence. During the study period in which HTL0018318 25 mg or placebo was combined with donepezil in subjects of cohort 2, there was an outbreak of a presumably viral gastrointestinal infection at the clinical research unit. When the gastrointestinal AEs related to the viral gastroenteritis were excluded from this analysis, no vomiting was reported in any of the treatment groups, and nausea was only reported by one subject who received placebo + donepezil and by one subject who received HTL0018138 15 mg + donepezil. Additionally, when excluding the viral gastroenteritis AEs, the number of AEs in the gastrointestinal disorders class was slightly higher for HTL0018318 + donepezil than for HTL0018318 alone (four AEs with placebo + donepezil vs. one AE for placebo alone; three AEs with HTL0018318 15 mg + donepezil vs. one AE with HTL0018318 15 mg alone; and four AEs with HTL0018318 25 mg + donepezil vs. three AEs with HTL0018318 25 mg alone).
3.3 Pharmacokinetics
3.3.1 HTL0018318 Pharmacokinetic Characteristics
Pharmacokinetic characteristics are shown in Tables 4 and 5. Plasma HTL0018318 concentrations increased immediately following dosing and after tmax (1.74–2.5 h); plasma concentrations declined in a biphasic manner. Pharmacokinetic steady state was reached for HTL0018318 on or before the fifth daily dose of HTL0018318.
3.3.2 HTL0018318 Accumulation
The mean ratio of the AUC0-tau of HTL0018318 after the fifth dose of HTL0018318 to AUC0–tau after the first dose of HTL0018318 was 1.27 for HTL0018318 15 mg and 1.23 for HTL0018318 25 mg. These ratios were comparable with co-administered donepezil: 1.23 for HTL0018318 15 mg and 1.21 for HTL0018318 25 mg.
The mean ratio of AUC0–tau of HTL0018318 after the fifth dose of HTL0018318 to the AUC0-–nf after the first dose of HTL0018318 was 1.04 following dosing with HTL0018318 15 mg and 1.06 after HTL0018318 25 mg. These ratios were comparable with co-administered donepezil: 1.04 for HTL0018318 15 mg and 1.03 for HTL0018318 25 mg.
3.3.3 Comparison of HTL0018318 Pharmacokinetic Characteristics in Combination with and Without Donepezil
The ratio of the pharmacokinetic parameters following the first dose of HTL0018318 + donepezil compared with HTL0018318 alone were 1.05 (90% CI 0.986–1.11) for Cmax, 1.01 (90% CI 0.793–1.28) for tmax, and 1.02 (90% CI 0.975–1.07) for AUC0–24. The ratios following the fifth dose of HTL0018318 were 1.04 for Cmax (90% CI 0.995–1.09), 0.974 (90% CI 0.744–1.28) for tmax, 1.00 (90% CI 0.969–1.03) for AUC0–tau, and 0.911 (90% CI 0.854–0.972) for Cmin.
3.3.4 Donepezil
The mean plasma donepezil concentration immediately before the first administration of HTL0018318 15 mg (15 h post donepezil dose) was 40.5 ng/mL (%CV 25.0), before HTL0018318 25 mg was 37.4 ng/mL (%CV 28.8), and before placebo was 36.1 ng/mL (%CV 29.6). Plasma donepezil concentrations after the 18th to 24th doses suggested that donepezil was at pharmacokinetic steady state by the 18th donepezil dose. The geometric mean ratios of the donepezil concentration at 4, 15, or 24 h post dosing with HTL0018318 at 15 or 25 mg on the first dose of HTL0018318 or at steady state versus donepezil plasma concentrations immediately before co-dosing (18th donepezil dose) was between 0.961 and 1.06, with the 90% CI including unity for all comparisons. The corresponding donepezil concentrations associated with dosing HTL0018318/placebo fell in the range of 0.915–1.06 with the 90% CI, including unity except at 24 h post-dose on day 1 of placebo administration where the ratio was 0.915 (90% CI 0.871–0.962).
4 Discussion
This randomized, double-blind, placebo-controlled trial in 42 (to deliver 36) healthy elderly subjects investigated the safety and tolerability and pharmacokinetics of repeated doses of HTL0018318 (15 or 25 mg) given without and in combination with donepezil 10 mg at steady state. An effect on tolerability could have been predicted since both donepezil and HTL0018318 enhance cholinergic activity. There was no a priori expectation of a pharmacokinetic drug–drug interaction.
AEs were reported by a high proportion of the subjects during the donepezil run-in phase. Multiple doses of HTL0018318 + donepezil were generally well tolerated. When HTL0018318 15 mg and placebo were combined with donepezil, a greater proportion of subjects reported AEs compared with HTL0018318 or placebo alone. This difference was likely caused by donepezil, as donepezil alone resulted in more AEs than HTL0018318 without donepezil. Since the percentage of subjects experiencing AEs with HTL0018318 25 mg without donepezil was comparable to that with donepezil alone, there was no difference when the treatments were combined.
The side effect profile observed in this study was comparable to that observed in the single ascending dose (SAD) and multiple ascending dose (MAD) studies with HTL0018318 [12, 14]. Only nausea and vomiting were reported more frequently than in the SAD and MAD study. During the study period in which HTL0018318 25 mg or placebo were dosed in combination with donepezil in subjects of cohort 2, there was an outbreak of a presumably viral gastrointestinal infection at the clinical research unit. This presumption was based on the clinical presentation of the symptoms and that it also affected the staff of the clinical research organisation and subjects receiving placebo. Additionally, individuals were affected one after the other and symptoms were not related to the timing of dosing.
Fewer AEs were reported after HTL0018318 15 mg + donepezil than after donepezil alone. A similar trend was observed in the placebo group. This may be explained by the high number of side effects that are associated with the start of intake of donepezil, which then decreases over time. In addition, the duration of the run-in period (20 days) was longer than the treatment period of HTL0018318 + donepezil (5 days).
The statistically significant increases in supine systolic blood pressure after administration of HTL0018318 25 mg + donepezil (1.6 mmHg) and after placebo + donepezil (1.7 mmHg) are considered to be of a small magnitude and not of clinical concern. The pulse rate data suggest that the combination of HTL0018318 and donepezil may decrease supine pulse rate but not standing pulse rate compared with HTL0018318 without donepezil. Accordingly, the physiological heart rate increment after standing up was greater in those who had received HTL0018318 + donepezil than in those receiving HTL0018318 without donepezil. However, these changes were similarly of small magnitude (up to 1.6 bpm) and of no clinical concern.
Increased saliva production was expected because of the mechanism of action of HTL0018318 [20] and because salivary hypersecretion has been described in other studies investigating M1 mAChR agonists [10, 21, 22], whereas it is not a common side effect of donepezil [23]. In the current study, the small changes in saliva production were not considered clinically important (Table S2 in the ESM).
Acetylcholine can elicit bronchoconstriction and mucous secretion by activating the M2 and M3 mAChRs on the airway smooth muscle and mucous glands. The M1 mAChRs might play a minor role as agonism of the M1 mAChRs at the postganglionic nerves facilitates acetylcholine release in the synaptic junction. This stimulates the M3 mAChRs, which contribute to bronchoconstriction and mucous secretion [24, 25]. The observed increase of FEV1/FVC in the current study, suggesting less constriction, is therefore not considered to be a pharmacological effect and not clinically important.
The M1 and M3 mAChRs play an essential role in the rapid eye movement phase during the sleep–wake cycle [26]. In the current study, no clinically relevant changes were observed on the LSEQ after administration of HTL0018318 alone or HTL0018318 + donepezil (Table S2 in the ESM).
The pharmacokinetics of HTL0018318 were well-characterized in plasma and urine. The characteristics were comparable to the pharmacokinetic data observed in previous studies [12, 14]. Median tmax (1.74–2.5 h) and mean half-life following the fifth dose (10.5–13.7 h) did not appear to change with respect to HTL0018318 dose level and co-dosing with donepezil. There was no apparent change in renal elimination of HTL0018318 due to changing HTL0018318 dose level or due to co-dosing with donepezil. Variability of the HTL0018318 plasma pharmacokinetic Cmax, AUC0–tau, and apparent elimination half-life was similar between the 15 and 25 mg dose groups and similar between the periods with and without donepezil co-dosing (between 12.0 and 39.2%). There appeared to be no trend in degree of accumulation related to HTL0018318 dose level or related to co-dosing with donepezil. Comparisons of the ratios for Cmax, tmax, AUC0–24, AUC0–tau, and Cmin (between 0.911 and 1.05) of HTL0018318 measured during the HTL0018318 dosing period with and without co-administration of donepezil indicate that donepezil does not have a meaningful impact on the pharmacokinetics of HTL0018318.
The plasma donepezil concentrations before the first administration of HTL0018318/placebo were considered to be therapeutic [27,28,29,30]. Comparisons of the plasma donepezil concentrations measured with and without co-administration of HTL0018318 indicate that HTL0018318 does not impact the pharmacokinetics of donepezil (mean ratios between 0.915 and 1.06).
A potential limitation of this study is the fixed treatment sequence: in all subjects, HTL0018318 + donepezil was administered first, then HTL0018318 alone was investigated. As explained in the methods section, this treatment sequence prevented unnecessary exposure to HTL0018318 in subjects who were not able to complete the donepezil run-in phase because of donepezil-related side effects. The impact of the sequence on the outcomes is considered to be low because subjects were blinded to treatment allocation, which is the most important factor in preventing bias in safety reporting. The unforeseen outbreak of a presumably viral gastrointestinal infection at the clinical research unit complicated the interpretation of the safety data. However, the clinical presentation allowed us to distinguish between the symptoms related to the presumably viral gastrointestinal infection and those that were drug related. In addition, we were able to collect more data on HTL0018318 25 mg because this dose level was also investigated in the third cohort.
5 Conclusion
Overall, HTL0018318 + donepezil in elderly healthy subjects was generally well tolerated, did not lead to clinical, safety, or pharmacokinetic concerns, and would be a viable combination treatment at these dose levels for the treatment of patients with AD.
References
Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochem Pharmacol. 2014;88(4):508–16. https://doi.org/10.1016/j.bcp.2014.01.015.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–80. https://doi.org/10.1002/ana.410300410.
Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, et al. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64(2):749–60. https://doi.org/10.1046/j.1471-4159.1995.64020749.x.
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–31. https://doi.org/10.3233/jad-132690.
Lanctot KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. CMAJ Can Med Assoc J (J Assoc Med Can) 2003;169(6):557–64.
Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem. 1995;64(4):1888–91.
Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci USA. 1996;93(24):13541–6.
Mash DC, Flynn DD, Potter LT. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer’s disease and experimental cholinergic denervation. Science. 1985;228(4703):1115–7.
Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–73.
Nathan PJ, Watson J, Lund J, Davies CH, Peters G, Dodds CM, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721–31. https://doi.org/10.1017/s1461145712000752.
Congreve M, Brown AJH, J C. Identification of novel Muscarinic M1 agonist HTL0018318 using structure based drug design. J Am Chem Soc. 2020. (in preparation).
Bakker C, Tasker T, Liptrot J, Hart EP, Klaassen ES, Doll RJ, et al. Safety, pharmacokinetics and exploratory pro-cognitive effects of HTL0018318, a selective M(1) receptor agonist, in healthy younger adult and elderly subjects: a multiple ascending dose study. Alzheimers Res Ther. 2021;13(1):87. https://doi.org/10.1186/s13195-021-00816-5.
fachinformation.srz.de. Donepezil—summary of product characteristics. http://fachinformation.srz.de/lp/aliudpharma/donepezilal5mg10mgfilmtabletten. Accessed 05 May 2016.
Bakker C, Tasker T, Liptrot J, Hart EP, Klaassen ES, Prins S, et al. First-in-man study to investigate safety, pharmacokinetics and exploratory pharmacodynamics of HTL0018318, a novel M(1) -receptor partial agonist for the treatment of dementias. Br J Clin Pharmacol. 2020. https://doi.org/10.1111/bcp.14710.
Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–8. https://doi.org/10.1111/j.2044-8341.1974.tb02285.x.
Parrott AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations—a review. Psychopharmacology. 1980;71(2):173–9.
Dai D, Yang H, Nabhan S, Liu H, Hickman D, Liu G, et al. Effect of itraconazole, food, and ethnic origin on the pharmacokinetics of ivosidenib in healthy subjects. Eur J Clin Pharmacol. 2019;75(8):1099–108. https://doi.org/10.1007/s00228-019-02673-6.
Sun L, McDonnell D, Yu M, Kumar V, von Moltke L. A phase I open-label study to evaluate the effects of rifampin on the pharmacokinetics of olanzapine and samidorphan administered in combination in healthy human subjects. Clin Drug Investig. 2019;39(5):477–84. https://doi.org/10.1007/s40261-019-00775-8.
Maekawa Y, Furuie H, Kato M, Myobatake Y, Kamiyama E, Watanabe A, et al. Effect of DS-8500a, a novel G protein-coupled receptor 119 agonist, on the pharmacokinetics of rosuvastatin and atorvastatin in healthy subjects. Clin Drug Investig. 2019;39(10):967–78. https://doi.org/10.1007/s40261-019-00825-1.
Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, et al. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17(7):1403–10.
Voss T, Li J, Cummings J, Farlow M, Assaid C, Froman S, et al. Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimer’s Dementia (New York, N Y). 2018;4:173–81. https://doi.org/10.1016/j.trci.2018.03.004.
Sramek JJ, Hurley DJ, Wardle TS, Satterwhite JH, Hourani J, Dies F, et al. The safety and tolerance of xanomeline tartrate in patients with Alzheimer’s disease. J Clin Pharmacol. 1995;35(8):800–6.
Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. The Cochrane database of systematic reviews. 2018;6:Cd001190. https://doi.org/10.1002/14651858.CD001190.pub3.
Buels KS, Fryer AD. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol. 2012;208:317–41. https://doi.org/10.1007/978-3-642-23274-9_14.
Castro JMdA, Resende RR, Mirotti L, Florsheim E, Albuquerque LL, Lino-dos-Santos-Franco A et al. Role of M2 muscarinic receptor in the airway response to methacholine of mice selected for minimal or maximal acute inflammatory response. BioMed Res Int. 2013;2013:805627. https://doi.org/10.1155/2013/805627.
Niwa Y, Kanda GN, Yamada RG, Shi S, Sunagawa GA, Ukai-Tadenuma M, et al. Muscarinic acetylcholine receptors Chrm1 and Chrm3 are essential for REM sleep. Cell Rep. 2018;24(9):2231-47.e7. https://doi.org/10.1016/j.celrep.2018.07.082.
Ota T, Shinotoh H, Fukushi K, Kikuchi T, Sato K, Tanaka N, et al. Estimation of plasma IC50 of donepezil for cerebral acetylcholinesterase inhibition in patients with Alzheimer disease using positron emission tomography. Clin Neuropharmacol. 2010;33(2):74–8. https://doi.org/10.1097/WNF.0b013e3181c71be9.
Shiraishi T, Kikuchi T, Fukushi K, Shinotoh H, Nagatsuka S, Tanaka N, et al. Estimation of plasma IC50 of donepezil hydrochloride for brain acetylcholinesterase inhibition in monkey using N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and PET. Neuropsychopharmacology. 2005;30(12):2154–61. https://doi.org/10.1038/sj.npp.1300759.
Valis M, Masopust J, Vysata O, Hort J, Dolezal R, Tomek J, et al. Concentration of donepezil in the cerebrospinal fluid of AD patients: evaluation of dosage sufficiency in standard treatment strategy. Neurotox Res. 2017;31(1):162–8. https://doi.org/10.1007/s12640-016-9672-y.
Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Archives of internal medicine. 1998;158(9):1021–31. https://doi.org/10.1001/archinte.158.9.1021.
Acknowledgements
This study was sponsored by Heptares Therapeutics Ltd. The authors thank Irene Morelli (York Bioanalytical Solutions, York, UK) for performing the bioanalysis of HTL0018318 and donepezil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Heptares Therapeutics Ltd.
Conflict of interest
Jan Liptrot, Steve Dickinson and Tim Tasker are currently paid employees of Sosei Heptares and have owned stock in the company. David M. Cross is a paid independent consultant for Sosei Heptares.
Ethics approval
This study was approved by the medical ethics review board of the foundation Beoordeling Ethiek Biomedisch Onderzoek (BEBO, Assen, The Netherlands).
Consent to participate
All subjects provided informed consent before participation.
Consent for publication
Not applicable
Availability of data and material
The datasets generated and/or analysed during the current study are filed in EudraCT and are not publicly available (in accordance with the regulations for phase I data). Further information is available from the corresponding author on reasonable request.
Code availability
The code that is used to model the data is available upon request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bakker, C., van der Aart, J., Labots, G. et al. Safety and Pharmacokinetics of HTL0018318, a Novel M1 Receptor Agonist, Given in Combination with Donepezil at Steady State: A Randomized Trial in Healthy Elderly Subjects. Drugs R D 21, 295–304 (2021). https://doi.org/10.1007/s40268-021-00352-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-021-00352-5