Abstract
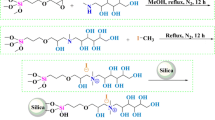
A precursor carboxy-silica support was introduced for grafting a retentive polar ligand, namely D-glucamine, for use in hydrophilic interaction liquid chromatography. This support was prepared by sequentially reacting 5 µm silica particles with vinyltrimethoxysilane and then thioglycolic acid. The carboxy-silica thus obtained was subsequently functionalized with D-glucamine by on-column reactions via a carbodiimide conjugation reaction. These reactions series, which are applied for the first time in HPLC column fabrication, yielded the D-glucamide-silica column. This polar column was evaluated for its hydrophilic interaction chromatography retention properties with derivatized mono- and oligosaccharides, phenolic and benzoic acid derivatives, nucleobases, nucleosides and nucleotides. The D-glucamide-silica column exhibited a good selectivity towards most of the hydrophilic solutes investigated. The derivatized sugars with the fluorescing 6-aminoquinoline tag were detected at a very low level of 7 × 10–8 mol/L, a fact that allowed the visualization of maltooligosaccharides up to a dp of 17.







Similar content being viewed by others
Availability of data and materials
Research data have been provided in the manuscript.
Code availability
Not applicable.
Abbreviations
- c-AMP:
-
Adenosine-2’:3’-cyclic monophosphate
- 6-AQ:
-
6-Aminoquinoline
- AIBN:
-
2,2’-Azobisisobutyronitrile
- CPS:
-
Chloropropylsilyl-silica
- CPTMS:
-
Chloropropyltrimethoxysilane
- EDAC:
-
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- c-GMP:
-
Guanosine-2’:3’-cyclic monophosphate
- p-HBA:
-
Para-Hydroxybenzoic acid
- pNP:
-
Para-Nitrophenyl
- c-UMP:
-
Uridine-2’:3’-cyclic monophosphate
References
Garcia-Gomez D, Rodríguez-Gonzalo E, Carabias-Martınez R (2013) Stationary phases for separation of nucleosides and nucleotides by hydrophilic interaction liquid chromatography. TRAC-Trends Anal Chem 47:111–128
Gama MR, Silva RGC, Collins CH, Bottoli CBG (2012) Hydrophilic interaction chromatography. TRAC-Trends. Anal Chem 37:48–60
Guo Y (2015) Recent progress in the fundamental understanding of hydrophilic interaction chromatography (HILIC). Analyst 140:6452–6466
Euerby MR, Hulse J, Petersson P, Vazhentsev A, Kassam K (2015) Retention modelling in hydrophilic interaction chromatography. Anal Bioanal Chem 407:9135–9152
Fu Q, Liang T, Zhang X, Du Y, Guo Z, Liang X (2010) Carbohydrate separation by hydrophilic interaction liquid chromatography on a ‘click’ maltose column. Carbohydr Res 345:2690–2697
Greco G, Letzel T (2013) Main interactions and influences of the chromatographic parameters in HILIC separations. J Chromatogr Sci 51:684–693
Dinh NP, Jonsson T, Irgum K (2011) Probing the interaction mode in hydrophilic interaction chromatography. J Chromatogr A 1218:5880–5891
Yoshida T (2004) Peptide separation by hydrophilic interaction chromatography: a review. J Biochem Bioph Meth 60:265–280
Berthod A, Chang SS, Kullman JP, Armstrong DW (1998) Practice and mechanism of HPLC oligosaccharide separation with a cyclodextrin bonded phase. Talanta 47:1001–1012
Buszewski B, Noga S (2012) Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique. Anal Bioanal Chem 402:231–247
Rathnasekara R, El Rassi Z (2017) Polar silica-based stationary phases. Part I - Singly and doubly layered sorbents consisting of TRIS-silica and chondroitin sulfate A-TRIS-silica for hydrophilic interaction liquid chromatography. Electrophoresis 38:1582–1591
Pereira AS, Giro´n AJ, Admasu E, Sandra P (2010) Green hydrophilic interaction chromatography using ethanol–water–carbon dioxide mixtures. J Sep Sci 33:834–837
Olsen BA (2001) Hydrophilic interaction chromatography using amino ad silica columns for the determination of polar pharmaceuticals and impurities. J Chromatogr A 913:113–122
Guo Y, Gaiki S (2011) Retention and selectivity of stationary phases for hydrophilic interaction chromatography. J Chromatogr A 1218:5920–5938
Jandera P (2011) Stationary and mobile phases in hydrophilic interaction chromatography: a review. Anal Chim Acta 692:1–25
Rathnasekara R, Khadka S, Jonnada M, El Rassi Z (2017) Polar and nonpolar organic polymer-based monolithic columns for capillary electrochromatography and high-performance liquid chromatography. Electrophoresis 38:60–79
Gunasena DN, El Rassi Z (2012) Organic monoliths for hydrophilic interaction electrochromatography/chromatography and immunoaffinity chromatography. Electrophoresis 33:251–261
Armstrong DW, Jin HL (1989) Evaluation of the liquid chromatographic separation of monosaccharides, disaccharides, trisaccharides, tetrasaccharides, deoxysaccharides and sugar alcohols with stable cyclodextrin bonded phase columns. J Chromatogr A 462:219–232
Schuster G, Lindner W (2011) Chocolate HILIC phases: development and characterization of novel saccharide-based stationary phases by applying non-enzymatic browning (Maillard reaction) on amino-modified silica surfaces. Anal Bioanal Chem 400:2539–2554
Bicker W, Wu J, Lämmerhofer M, Lindner W (2008) Hydrophilic interaction chromatography in nonaqueous elution mode for separation of hydrophilic analytes on silica-based packings with noncharged polar bondings. J Sep Sci 31:2971–2987
Kawachi Y, Ikegami T, Takubo H, Ikegami Y, Miyamoto M, Tanaka N (2011) Chromatographic characterization of hydrophilic interaction liquid chromatography stationary phases: hydrophilicity, charge effects, structural selectivity, and separation efficiency. J Chromatogr A 1218:5903–5919
Alharthi S, El Rassi Z (2018) Poly(2-carboxyethyl acrylate-co-ethylene glycol dimethacrylate) monolithic precursor. Part II. Carbodiimide assisted post-polymerization modification with tris and d-Glucamine for use in hydrophilic interaction capillary liquid chromatography. J Liq Chrom & Relat Technol 41:684–691
Peng X, Liu T, Ji S, Feng Y (2013) Preparation of a novel carboxyl stationary phase by “thiol-ene” click chemistry for hydrophilic interaction chromatography. J Sep Sci 36:2571–2577
Paranamana N, El Rassi Z (2020) Precursor carboxy-silica for functionalization with interactive ligands. I. Carbodiimide-assisted preparation of silica-bonded stationary phases with octadecyl, naphthyl, and anthracenyl ligands: comparison of their selectivity and retentivity. J Sep Sci 43:4424–4433
Nashabeh W, El Rassi Z (1992) Capillary zone electrophoresis of linear and branched oligosaccharides. J Chromatogr A 600:279–287
Zhuravlev LT, Potapov VV (2006) Density of silanol groups on the surface of silica precipitated from a hydrothermal solution. Russ J Phys Chem 80:119–1128
Colin H, Guiochon G, Yun Z, Diez-Masa JC, Jandera J (1983) Selectivity for homologous series in reversed-phase LC: investigation of non-specific selectivity. J Chromatogr Sci 21:179–184
Smith JT, Nashabeh W, El Rassi Z (1994) Micellar electrokinetic capillary chromatography with in situ charged micelles.1. Evaluation of N-D-gluco-N-methylalkanamide surfactants as anionic borate complexes. Anal Chem 66:1119–1133
Brynes PJ, Bevilacqua P, Green A (1981) 6-aminoquinoline as a fluorogenic leaving group in peptide cleavage reactions: a new fluorogenic substrate for chymotrypsin. Anal Biochem 116:408–413
Mallik AK, Guragain S, Rahman MM, Takafuji M, Ihara H (2019) L-Lysine-derived highly selective stationary phases for hydrophilic interaction chromatography: effect of chain length on selectivity, efficiency, resolution, and asymmetry. Sep Sci Plus 2:42–50
Tyteca E, Guillarme D, Desmet G (2014) Use of individual retention modeling for gradient optimization in hydrophilic interaction chromatography: separation of nucleobases and nucleosides. J Chromatogr A 1368:125–131
Rathnasekara R, El Rassi Z (2017) Polar silica-based stationary phases. Part II- Neutral silica stationary phases with surface bound maltose and sorbitol for hydrophilic interaction liquid chromatography. J Chromatogr A 1508:24–32
Svec F, Fréchet J (1992) Continuous rods of macroporous polymer as high performance liquid chromatography separation media. Anal Chem 64:820–822
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NP carried out the experimental studies, analysis, data interpretation, and wrote the manuscript. ZER supervised the entire work and edited the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest/competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paranamana, N., El Rassi, Z. Precursor Carboxy-silica for Functionalization With Interactive Ligands. II. Carbodiimide Assisted Preparation of Silica Bonded Stationary Phases with D-glucamine for Hydrophilic Interaction Liquid Chromatography. Chromatographia 84, 781–791 (2021). https://doi.org/10.1007/s10337-021-04062-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04062-7