The Urinary Hormonal State of Cats Associated With Social Interaction With Humans
- 1Department of Human and Animal-Plant Relationships, Graduate School of Agriculture, Tokyo University of Agriculture, Atsugi, Japan
- 2Department of Animal Science and Biotechnology, Azabu University School of Veterinary Medicine, Sagamihara, Japan
- 3Department of Human and Animal-Plant Relationships, Faculty of Agriculture, Tokyo University of Agriculture, Atsugi, Japan
- 4Department of Animal Science, Faculty of Agriculture, Tokyo University of Agriculture, Atsugi, Japan
Research to assess the relationship between cats and humans is in a nascent stage. Some studies have assessed the stress status in cats using physiological indicators, such as the cortisol hormone, but have not focused on the social interaction with humans. Moreover, the role of oxytocin secretion in the relationship between cats and humans remains unclear. In this study, we determined the possibility of quantifying the urinary concentration of oxytocin in cats and assessed the effects of social contact with humans on the levels of urinary oxytocin and cortisol metabolite. Four cats were subjected to two conditions, namely, social (control), and non-social (no social contact with humans) conditions. The levels of cortisol and oxytocin metabolite in urine samples from the cats in both conditions were determined using enzyme-linked immunosorbent assays. The urinary concentrations of cortisol and oxytocin under the non-social condition were significantly higher than those under the social condition. In addition, the concentration of oxytocin significantly correlated with that of cortisol in cats under the non-social condition. In this study, it was possible to quantify the concentration of oxytocin in the urine of cats, and the obtained results suggest that cats recognize the social interaction with humans as important. This information might contribute to the establishment of an assessment method for the welfare of cats and might help in clarifying the relationship between cats and humans.
Introduction
The number of cats exceeds that of dogs in Japan (1), and this trend is common worldwide (2). The life expectancy of cats (3, 4) as well as of humans (5) has increased. To enhance the relationship between cats and humans, more information related to the human–cat interaction is needed.
There is a large and increasing number of questionnaires related to the human–animal interaction (HAI) (6). A questionnaire is an essential subjective indicator to assess the relationships between cats and humans. However, Rodriguez et al. (7) has highlighted the need to incorporate methodologically rigorous designs, combining both subjective and objective outcome measures, for developing the field of HAI. Thus, objective measurements, such as behavioral observation and physiological assessment, are also fundamental indicators in the field of HAI research. Behavioral observation, for example, the development of an ethogram (8, 9), is frequently used to assess the relationships between humans and cats. The cat stress score is a well-known observational assessment scale for evaluating the stress status in cats (10). Several studies on shelter cats used this assessment scale (11, 12); however, these studies have focused on the welfare of the cats, and not their relationship with humans.
Several physiological indicators, such as the heart rate variability and blood pressure, are mainly used in the field of veterinary research to assess the clinical conditions of cats (13, 14). Cortisol is a steroid hormone released to help cope with an acute stressor (15); it is, therefore, a useful indicator of the stress status in cats. Blood is a valid sample for quantifying the concentration of cortisol (16). However, procurement of this sample type is accompanied by physical confirmation (e.g., holding of the body) and an invasive procedure; thus, researchers should have confirmation sample types that can be collected non-invasively. For example, feces (17, 18) and hair (18) samples have been used to measure the concentration of cortisol. Especially in the field of HAI research, urine samples are useful because of the ease of collection. These studies have been conducted under various conditions, for example, in shelter (19, 20), laboratory (21), and house (22). Nevertheless, these studies were focused on the welfare of cats, not on their social interaction and relationship with humans.
Recently, oxytocin has received much attention in the field of HAI research. Oxytocin has variable functions, for example, in stress reduction, such as in decreasing the cortisol concentration and blood pressure (23), promoting well-being (24), and increasing social behavior (25). Rault et al. (26) mentioned that oxytocin is an essential indicator of psychological and social well-being in domesticated animals. Furthermore, oxytocin has a function related to pregnancy and uterine contractions (27), and is related to the construction of attachment relationships between infants and mothers (28). Some studies have shown relationships between dogs and humans, similar to those of infants and mothers (29).
Mutual interaction between dogs and their owners causes the secretion of oxytocin from their bodies (30, 31). Pet owners develop attachment not only with their dogs but also with cats (32). Therefore, oxytocin secretion may be a key factor in creating a bond between cats and humans. However, Potter and Mills (33) suggested that the attachment between cats and their owners is not transparent. Additionally, it is unclear whether oxytocin secretion is associated with the construction of a bond between cats and humans. To understand the genuine relationships between cats and humans, it is necessary to conduct a study focused on oxytocin. Blood oxytocin concentration in cats has been assessed by enzyme-linked immunosorbent assay (ELISA) (34). However, blood sampling can be quite stressful for the animals, and the evaluation of the correct physiological values is difficult. The content of urine is filtered from the blood, accumulated in a certain amount, and then naturally expelled. Therefore, urinary analysis may be an optimal non-invasive method for assessing these physiological conditions.
The purpose of this study was to determine whether the feline urinary cortisol and oxytocin metabolite concentrations could be quantified by ELISA. In addition, to clarify the relationship between cats and humans, we examined whether social contact with humans affects the concentration of these hormones in cats.
Method
Ethics Statement
The experiments performed in this study were approved by the Animal Experiment Ethics Committee (approval number: 1301312) at the Tokyo University of Agriculture in accordance with the World Medical Association's Declaration of Helsinki.
Test Animals
The experiment was performed on four cats (A: 3-year-old, male, mix; B: 6-year-old, male; C: 10-year-old, female, Ragdoll; D: 3-year-old, female, mix). All the cats had always lived in a laboratory room (7 × 7 m) like a house cat. The cats freely spent time in the same room and were individually kept in a three-tier cage (93 × 63 × 178 cm) during the nighttime. Additionally, a caretaker looked after the cats as a house cat every day, ensuring proper feeding, physical care, playing, clicker training, physical contact (touching, petting, and grooming), and oral communication (calling and talking).
Assay Methods
Collection of Urine Samples
To ensure the welfare of the cats, we adopted a non-invasive method of urine collection by natural urination. Additionally, we collected spot urine samples, instead of pooled samples, each time from the tray in litter boxes and transferred them directly to plastic 2-ml centrifuge tubes. The samples were kept frozen at −80°C until analysis. For quantification, the supernatant obtained after centrifugation of the urine samples at 1,661 × g for 15 min at 4°C was used.
Quantification of Cortisol in Urine Samples
Urinary cortisol metabolite concentration was determined using the DetectX® Cortisol Enzyme Immunoassay Kit (K003—H5W, Arbor Assays LLC, USA; goat anti-mouse IgG) used in previous studies (35, 36). The assay standard curve ranged from 50 to 3,200 pg/ml, and the assay sensitivity was 27.6 pg/ml. The urine samples were diluted 10-fold with the assay buffer. The intra-assay coefficient of variation (CV) for the cortisol assay was 4.10%, and the inter-assay CV was 5.25%.
Quantification of Oxytocin in Urine Samples
The urine samples were extracted with a Hyper Sep C18 column (3 ml/200 g, Thermo Fisher Scientific, Tokyo), as described by Finkenwirth et al. (37). Previous studies successfully quantified the urinary metabolite concentrations in dogs, wolves, and humans (38, 39). The C18 column was conditioned by washing three times with 3 ml of 100% methanol and then three times with 3 ml of distilled water. A mixture of 1 ml of urine sample and 10 μl of phosphoric acid was transferred to the column. The columns were washed with 3 ml of 10% acetonitrile and 0.1% trifluoroacetic acid. Samples were then eluted with 1 ml of 80% acetonitrile. The eluted samples were dried using an evaporator. The dried samples were reconstituted in 1 ml of assay buffer provided with the kit and used for determining the metabolite concentration of oxytocin. Urinary oxytocin metabolite concentration was determined using the DetectX® oxytocin Enzyme Immunoassay Kit (K048 - H5, Arbor Assays LLC; goat anti-rabbit IgG) used in previous studies (40, 41). The assay standard curve ranged from 16.38 to 10,000 pg/ml, the assay sensitivity was 17.0 pg/ml, the intra-assay CV for the oxytocin assay was 4.25%, and the inter-assay CV was 4.59%.
Quantification of Creatinine in Urine Samples
Throughout the experiments, the four cats had free access to drinking water; thus, the water intake of individual cats varied daily. Therefore, the urinary hormone concentrations need to be corrected by urinary creatinine to account for the quantity of water in the sample. All oxytocin and cortisol levels were described as pg/mg creatinine (Cre). The concentration of creatinine was measured by the Jaffe reaction using 96-well microplates (3881–096, Iwaki, Japan). After the reaction, the optical density was read at 490 nm using a microplate reader.
Experimental Protocol
The experiment was performed under two conditions: social condition (SC) and non-social condition (NSC). In SC, urine samples were continuously collected for 3 days. In NSC, arrangements were made such that the caretaker engaged in minimum necessary care (e.g., feeding and managing the environment), excluding social contact (e.g., physical care, playing, clicker training, physical contact, and oral communication) for 3 days. The interval between the experiments under the two conditions was 2 weeks. All cats were fed a constant amount of food throughout the experiment to negate its effects on urinary hormone metabolite concentrations.
Statistical Analyses
We excluded outliers, defined as 1.5 × interquartile range (IQR), from the analysis. The difference in the mean hormone concentration during SC and NSC was determined using the Welch's t-test or Mann–Whitney U-test. The effect sizes were calculated by Cohen's d. Using Spearman's rank correlation coefficient, we assessed the correlation between cortisol and oxytocin concentrations. Statistical significance was set at p < 0.05. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Japan). There was no outliner in the cortisol assay, whereas there were three outliers in both SC and NSC in the oxytocin assay.
Results
We collected 54 urine samples and performed the different quantification assays. The mean cortisol metabolite concentrations in SC and NSC were 5,433.40 ± 1,805.02 (n = 25, range 1,843.53–9,528.73) and 6,339.98 ± 1,908.95 (n = 29, range 3,531.81–11,048.50) pg/mg•Cre, respectively (Figure 1A). The mean oxytocin metabolite concentrations in SC and NSC were 115.72 ± 9.28 (n = 22, range 31.62–203.48) and 193.06 ± 82.57 (n = 26, range 61.63–383.91) pg/mg•Cre, respectively (Figure 1B).
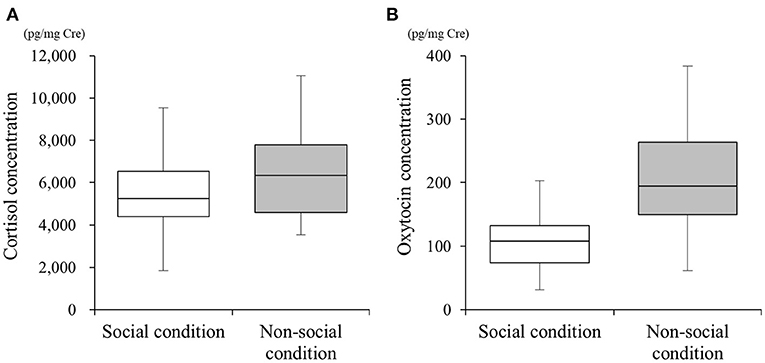
Figure 1. Differences in urinary cortisol (A) and oxytocin (B) concentrations under social (SC) and non-social (NSC) conditions. Box plots show the interquartile range (IQR) for each condition, with whiskers extending to 1.5 × the IQR.
There was a significant difference in the cortisol concentration between SC and NSC conditions in all 54 samples (Figure 2A, p < 0.05; Cohen's d: 0.49). However, no differences were found when analyzing the samples collected from each individual: A (p > 0.05; Cohen's d: 0.98), B (p > 0.05; Cohen's d: 0.44), C (p > 0.05; Cohen's d: 0.02), and D (p > 0.05; Cohen's d: 0.96).
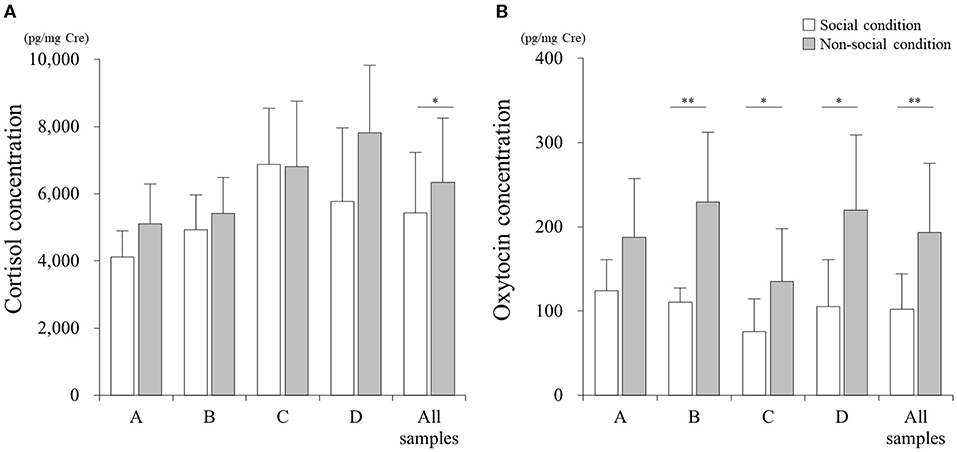
Figure 2. Comparisons of cortisol (A) and oxytocin (B) concentrations between social (SC) and non-social (NSC) conditions. *p < 0.05, **p < 0.01.
The concentration of oxytocin in NSC significantly increased relative to that in SC for all the samples (Figure 2B, p < 0.01; Cohen's d: 1.39), and samples belonging to cats B (p < 0.01; Cohen's d: 1.98), C (p < 0.05; Cohen's d: 1.14), and D (p < 0.05; Cohen's d: 1.54); however, there was no difference for cat A (p > 0.05; Cohen's d: 1.13).
In all the samples, there was a significant correlation between cortisol and oxytocin concentrations (Figure 3A, r = 0.40, p < 0.01). In SC, the concentration of cortisol did not correlate with that of oxytocin (Figure 3B, r = 0.07); however, in NSC, cortisol concentration significantly correlated with that of oxytocin (Figure 3C, r = 0.45, p < 0.01).
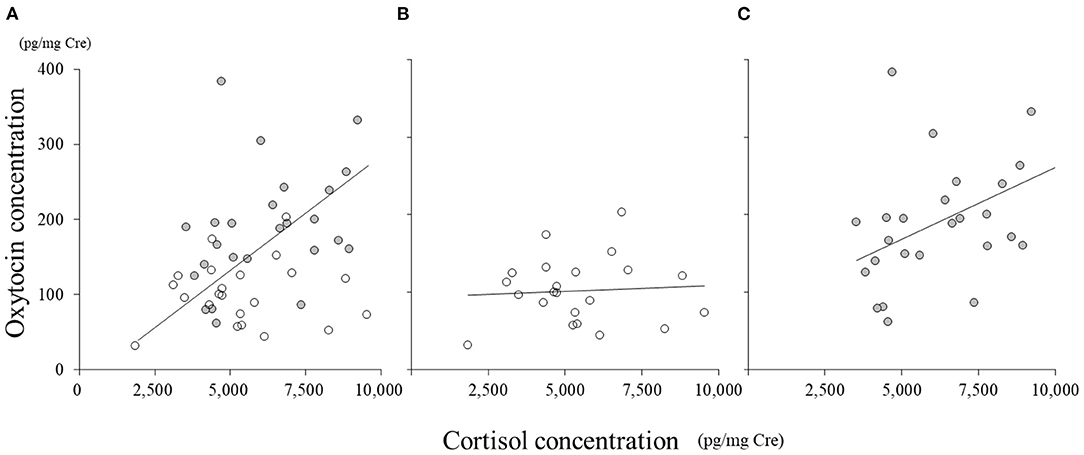
Figure 3. Correlations between oxytocin and cortisol concentrations. (A) All samples; (B) social condition (SC); (C) non-social condition (NSC).
Discussion
We showed that it is possible to quantify the urinary metabolite concentrations of hormones in cats using ELISA; hitherto, there has only been one report (34) on the quantification of the blood oxytocin concentration in cats. Measurement of both cortisol and oxytocin might help in accurately assessing the physiological status of cats because oxytocin influences the activities of the hypothalamic–pituitary–adrenal axis and autonomic nervous system, as does cortisol (23). Measurement of not only cortisol but also of oxytocin is a useful method to accurately understand the physical status of house cats; therefore, our results are of great importance in the field of HAI.
It is notable that we used the natural spot of urination of cats. Because urine sampling through an invasive method using catheters might have negative effects on the welfare of cats, we adopted a non-invasive method. Thus, methods for collection of samples for physiological assessment of cats under different situations (e.g., laboratory cats, shelter cats, stray cats, and household cats) should be expanded. Additionally, in this study, we focused on urinary metabolite concentration, and not locally produced and circulating hormones. Urine metabolites accumulate in the bladder for a long time; thus, urinary metabolite concentration reflects the long-term physiological condition. Urinary metabolite hormone concentrations correlate with circulating hormone concentration (42). This is an advantage in assessing the basal and long-term physiological states, and not just temporary and short-term states. The purpose of this study was to determine the physiological state of cats for 3 days; therefore, metabolite concentrations served as reasonable indicators. Future studies should focus on developing quantification methods for urinary hormone metabolites as assessment tools for the welfare of cats.
There is controversy regarding the immune-reactivity of urinary oxytocin metabolite in ELISA. Previous studies in dogs, wolves, and humans reported that urinary oxytocin metabolite has two peaks of immune-reactivity although the oxytocin metabolite concentration can be quantified (38, 39, 42). Moreover, a previous study reported no relationship between OT in the plasma and urinary samples in humans (43). The findings of this study should be interpreted carefully. In the future, it is necessary to verify the immune reactivity of urinary oxytocin in cats.
In the present study, the metabolite concentrations of both cortisol and oxytocin under NSC were higher than those under SC. Acute stressors induce the secretion of cortisol; thus, cats might perceive the interception of social communication with humans as a stressful event. In previous studies, interaction with humans has positive physiological and behavioral effects on shelter cats (44, 45). Our results possibly support the results of these studies from the perspective of hormonal change. Additionally, oxytocin concentrations in three out of four cats were different between SC and NSC conditions, although the difference for cortisol concentrations was not confirmed. Oxytocin has the functions not only to inhibit and reduce stress (46, 47) but also to promotes social behavior (48–50). Oxytocin might have been secreted in cats seeking social interaction with humans; therefore, we believe that cats recognize interactions with humans as important. Moreover, in NSC, but not in SC, urinary cortisol concentration was significantly correlated with oxytocin. The basal plasma concentrations of oxytocin and cortisol have a positive correlation only when the experimental situation causes anticipation of stress or a novel situation (51). Our results show that cats might have perceived NSC as a stressful event, and as such, the concentration of urinary oxytocin would be correlated with that of urinary cortisol under social stress conditions, but not under normal conditions.
It is not easy to interpret the results of this study. At first, oxytocin has variable physiological functions; thus, the rigorous causal relationship for consequence is still unclear. Second, there is evidence showing the negative correlation between circulating oxytocin and cortisol (52), namely, oxytocin has the function to inhibit the activity of cortisol. The reverse consequence of this study may be explained as a difference in the period of interest. The phenomenon that oxytocin decreases the cortisol concentration might occur following both cortisol and oxytocin temporal increase. In the case of the result of the above whole phenomenon, both the urinary oxytocin and cortisol metabolite concentration might be high levels; therefore, it is a possible explanation that both urinary oxytocin and cortisol of cats showed high levels in the social stress condition. However, it is very difficult to judge the conclusion by only this study. This study had a potential limitation, as only four cats were included in this study, and the number of urinary samples collected was small. Moreover, the cats were only kept for 3 days under NSC considering the welfare of the cats. In the future, it is required that more cats and urine samples are analyzed, and deeply discuss the relationship between cortisol and oxytocin.
Herein, we demonstrated the possibility of quantifying the urinary metabolite concentration of oxytocin in cats. Moreover, the urinary metabolite concentrations of oxytocin and cortisol in cats were found to be influenced by social interaction with humans. The results of the present study should contribute to the development of strategies for the welfare of cats and should provide a new perspective on the social relationship between cats and humans.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by Animal Experiment Ethics Committee at the Tokyo University of Agriculture.
Author Contributions
TN performed the experiments and analyzed the data. All authors planned the experiments and wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Editage (www.editage.com) for the English-language editing.
References
1. Japan Pet Food Association. The breeding rate and number of breeding of dogs and cats. Japan Pet Food Assoc. (2020). Available online at: https://petfood.or.jp/data/chart2020/3.pdf (accessed: January 14, 2021)
2. Pet Secure. Worldwide Pet Ownership Statistics|Most Common Pets Around the World. (2016). Available online at: https://www.petsecure.com.au/pet-care/a-guide-to-worldwide-pet-ownership/ (accessed: January 9, 2021).
4. Cozzi B, Ballarin C, Mantovani R, Rota A. Aging and veterinary care of cats, dogs, and horses through the records of three university veterinary hospitals. Front Vet Sci. (2017) 4:14. doi: 10.3389/fvets.2017.00014
5. United Nations. World Population Ageing 2019: Highlights. (2019). Available online at: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed: January 9, 2021).
6. Wilson CC, Netting FE. The status of instrument development in the human–animal interaction field. Anthrozoos. (2012) 25:s11–55. doi: 10.2752/175303712X13353430376977
7. Rodriguez KE, Guérin NA, Gabriels RL, Serpell JA, Schreiner PJ, O'haire ME. The state of assessment in human-animal interaction research. Human-Animal Interact Bull. (2018) 6:63–81. Available online at: https://www.vet.purdue.edu/chab/ohaire/files/documents/HAI_2018_HAIB_Rodriguez.pdf
8. Ellis SLH, Wells DL. The influence of visual stimulation on the behaviour of cats housed in a rescue shelter. Appl Anim Behav Sci. (2008) 113:166–74. doi: 10.1016/j.applanim.2007.11.002
9. Ellis SLH, Thompson H, Guijarro C, Zulch HE. The influence of body region, handler familiarity and order of region handled on the domestic cat's response to being stroked. Appl Anim Behav Sci. (2015) 173:60–7. doi: 10.1016/j.applanim.2014.11.002
10. Kessler MR, Turner DC. Stress and adaptation of cats (Felis silvestris catus) housed singly, in pairs and in groups in boarding catteries. Anim Welf. (1997) 6:243–54.
11. van der Leij WJR, Selman LDAM, Vernooij JCM, Vinke CM. The effect of a hiding box on stress levels and body weight in Dutch shelter cats; a randomized controlled trial. PLoS ONE. (2019) 14:e0223492. doi: 10.1371/journal.pone.0223492
12. Vinke CM, Godijn LM, van der Leij WJR. Will a hiding box provide stress reduction for shelter cats? Appl Anim Behav Sci. (2014) 160:86–93. doi: 10.1016/j.applanim.2014.09.002
13. Khor KH, Shiels IA, Campbell FE, Greer RM, Rose A, Mills PC. Evaluation of a technique to measure heart rate variability in anaesthetised cats. Vet J. (2014) 199:229–35. doi: 10.1016/j.tvjl.2013.11.006
14. Sugimoto K, Kawase N, Aoki T, Fujii Y. Effcts of dehydration on echocardiographic diastolic parameters in healthy cats. J Vet Sci. (2019) 20:e18. doi: 10.4142/jvs.2019.20.e18
15. Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci Biobehav Rev. (2016) 68:651–68. doi: 10.1016/j.neubiorev.2016.06.038
16. Stella J, Croney C, Buffington T. Effects of stressors on the behavior and physiology of domestic cats. Appl Anim Behav Sci. (2013) 143:157–63. doi: 10.1016/j.applanim.2012.10.014
17. Ellis JJ, Protopapadaki V, Stryhn H, Spears J, Cockram MS. Behavioural and faecal glucocorticoid metabolite responses of single caging in six cats over 30 days. Vet Rec Open. (2014) 1:e000056. doi: 10.1136/vropen-2014-000056
18. Finkler H, Terkel J. The relationship between individual behavioural styles, dominance rank and cortisol levels of cats living in urban social groups. Appl Anim Behav Sci. (2015) 173:22–8. doi: 10.1016/j.applanim.2015.04.016
19. McCobb EC, Patronek GJ, Marder A, Dinnage JD, Stone MS. Assessment of stress levels among cats in four animal shelters. J Am Vet Med Assoc. (2005) 226:548–55. doi: 10.2460/javma.2005.226.548
20. Uetake K, Goto A, Koyama R, Kikuchi R, Tanaka T. Effects of single caging and cage size on behavior and stress level of domestic neutered cats housed in an animal shelter. Anim Sci J. (2013) 84:272–4. doi: 10.1111/j.1740-0929.2012.01055.x
21. Carlstead K, Brown JL, Strawn W. Behavioral and physiological correlates of stress in laboratory cats. Appl Anim Behav Sci. (1993) 38:143–58. doi: 10.1016/0168-1591(93)90062-T
22. Lichtsteiner M, Turner DC. Influence of indoor-cat group size and dominance rank on urinary cortisol. Anim Welf. (2008) 17:215–37. Available online at: https://psycnet.apa.org/record/2008-11318-001
23. Buemann B, Uvnäs-Moberg K. Oxytocin may have a therapeutical potential against cardiovascular disease. possible pharmaceutical and behavioral approaches. Med Hypotheses. (2020) 138:109597. doi: 10.1016/j.mehy.2020.109597
24. Ito E, Shima R, Yoshioka T. A novel role of oxytocin: oxytocin-induced well-being in humans. Biophys Physicobiol. (2019) 16:132–9. doi: 10.2142/biophysico.16.0_132
25. Quintana DS, Guastella AJ. An allostatic theory of oxytocin. Trends Cogn Sci. (2020) 24:515–28. doi: 10.1016/j.tics.2020.03.008
26. Rault J-L, van den Munkhof M, Buisman-Pijlman FTA. Oxytocin as an indicator of psychological and social well-being in domesticated animals: a critical review. Front Psychol. (2017) 8:1521. doi: 10.3389/fpsyg.2017.01521
27. Uvnäs-Moberg K, Ekström-Bergström A, Berg M, Buckley S, Pajalic Z, Hadjigeorgiou E, et al. Maternal plasma levels of oxytocin during physiological childbirth - a systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth. (2019) 19:285. doi: 10.1186/s12884-019-2365-9
28. Scatliffe N, Casavant S, Vittner D, Cong X. Oxytocin and early parent-infant interactions: a systematic review. Int J Nurs Sci. (2019) 6:445–53. doi: 10.1016/j.ijnss.2019.09.009
29. Ryan MG, Storey AE, Anderson RE, Walsh CJ. Physiological indicators of attachment in domestic dogs (Canis familiaris) and their owners in the strange situation test. Front Behav Neurosci. (2019) 13:162. doi: 10.3389/fnbeh.2019.00162
30. Nagasawa M, Kikusui T, Onaka T, Ohta M. Dog's gaze at its owner increases owner's urinary oxytocin during social interaction. Horm Behav. (2009) 55:434–41. doi: 10.1016/j.yhbeh.2008.12.002
31. Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, et al. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science. (2015) 348:333–6. doi: 10.1126/science.1261022
32. Vitale KR, Behnke AC, Udell MAR. Attachment bonds between domestic cats and humans. Curr Biol. (2019) 29:R864–5. doi: 10.1016/j.cub.2019.08.036
33. Potter A, Mills DS. Domestic cats (Felis silvestris catus) do not show signs of secure attachment to their owners. PLoS ONE. (2015) 10:e0135109. doi: 10.1371/journal.pone.0135109
34. Bienboire-Frosini C, Chabaud C, Cozzi A, Codecasa E, Pageat P. Validation of a commercially available enzyme immunoassay for the determination of oxytocin in plasma samples from seven domestic animal species. Front Neurosci. (2017) 11:524. doi: 10.3389/fnins.2017.00524
35. Brand CM, Boose KJ, Squires EC, Marchant LF, White FJ, Meinelt A, et al. Hair plucking, stress, and urinary cortisol among captive bonobos (Pan paniscus). Zoo Biol. (2016) 35:415–22. doi: 10.1002/zoo.21320
36. Righi C, Menchetti L, Orlandi R, Moscati L, Mancini S, Diverio S. Welfare assessment in shelter dogs by using physiological and immunological parameters. Animals. (2019) 9:340. doi: 10.3390/ani9060340
37. Finkenwirth C, van Schaik C, Ziegler TE, Burkart JM. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiol Behav. (2015) 151:246–51. doi: 10.1016/j.physbeh.2015.07.034
38. Schaebs FS, Marshall-Pescini S, Range F, Deschner T. Analytical validation of an Enzyme Immunoassay for the measurement of urinary oxytocin in dogs and wolves. Gen Comp Endocrinol. (2019) 281:73–82. doi: 10.1016/j.ygcen.2019.05.015
39. Schaebs FS, Wirobski G, Marshall-Pescini S, Range F, Deschner T. Validation of a commercial Enzyme Immunoassay to assess urinary oxytocin in humans. Endocr Connect. (2021) 10:290–301. doi: 10.1530/EC-20-0583
40. Wirobski G, Range F, Schaebs FS, Palme R, Deschner T, Marshall-Pescini S. Endocrine changes related to dog domestication: comparing urinary cortisol and oxytocin in hand-raised, pack-living dogs and wolves. Horm Behav. (2021) 128:104901. doi: 10.1016/j.yhbeh.2020.104901
41. Leeds A, Good J, Schook MW, Dennis PM, Stoinski TS, Willis MA, et al. Evaluating changes in salivary oxytocin and cortisol following positive reinforcement training in two adult male western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. (2020) 39:51–5. doi: 10.1002/zoo.21524
42. Ziegler TE. Measuring peripheral oxytocin and vasopressin in nonhuman primates. Am J Primatol. (2018) 80:e22871. doi: 10.1002/ajp.22871
43. Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. (2011) 14:752–61. doi: 10.1111/j.1467-7687.2010.01021.x
44. Gourkow N, Hamon SC, Phillips CJC. Effect of gentle stroking and vocalization on behaviour, mucosal immunity and upper respiratory disease in anxious shelter cats. Prev Vet Med. (2014) 117:266–75. doi: 10.1016/j.prevetmed.2014.06.005
45. Gourkow N, Phillips CJC. Effect of cognitive enrichment on behavior, mucosal immunity and upper respiratory disease of shelter cats rated as frustrated on arrival. Prev Vet Med. (2016) 131:103–10. doi: 10.1016/j.prevetmed.2016.07.012
46. Dief AE, Sivukhina EV, Jirikowski GF. Oxytocin and stress response. Open J Endocr Metab Dis. (2018) 08:93–104. doi: 10.4236/ojemd.2018.83010
47. Kapur A, Kapur V. The multifarious oxytocin: a review. Int J Res Med Sci. (2019) 7:1992. doi: 10.18203/2320-6012.ijrms20191717
48. Caldwell HK. Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist. (2017) 23:517–28. doi: 10.1177/1073858417708284
49. Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. (2009) 30:534–47. doi: 10.1016/j.yfrne.2009.05.004
50. Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. (2010) 48:179–84. doi: 10.1016/j.neuropsychologia.2009.09.003
51. Brown CA, Cardoso C, Ellenbogen MA. A meta-analytic review of the correlation between peripheral oxytocin and cortisol concentrations. Front Neuroendocrinol. (2016) 43:19–27. doi: 10.1016/j.yfrne.2016.11.001
Keywords: cats, humans, social interaction, cortisol, oxytocin, urinary
Citation: Nagasawa T, Ohta M and Uchiyama H (2021) The Urinary Hormonal State of Cats Associated With Social Interaction With Humans. Front. Vet. Sci. 8:680843. doi: 10.3389/fvets.2021.680843
Received: 15 March 2021; Accepted: 23 June 2021;
Published: 26 July 2021.
Edited by:
Emma Kathryn Grigg, University of California, Davis, United StatesReviewed by:
Lynette Arnason Hart, University of California, Davis, United StatesDennis Clair Turner, Institute for applied Ethology and Animal Psychology, Switzerland
Copyright © 2021 Nagasawa, Ohta and Uchiyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidehiko Uchiyama, h3uchiya@nodai.ac.jp
 Takumi Nagasawa
Takumi Nagasawa Mitsuaki Ohta1,2
Mitsuaki Ohta1,2  Hidehiko Uchiyama
Hidehiko Uchiyama