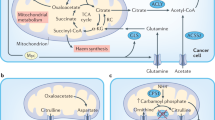
Abstract
Colorectal cancer (CRC) requires massive iron stores, but the complete mechanisms by which CRC modulates local iron handling are poorly understood. Here, we demonstrate that hepcidin is activated ectopically in CRC. Mice deficient in hepcidin specifically in the colon tumour epithelium, compared with wild-type littermates, exhibit significantly diminished tumour number, burden and size in a sporadic model of CRC, whereas accumulation of intracellular iron by deletion of the iron exporter ferroportin exacerbates these tumour parameters. Metabolomic analysis of three-dimensional patient-derived CRC tumour enteroids indicates a prioritization of iron in CRC for the production of nucleotides, which is recapitulated in our hepcidin/ferroportin mouse CRC models. Mechanistically, our data suggest that iron chelation decreases mitochondrial function, thereby altering nucleotide synthesis, whereas exogenous supplementation of nucleosides or aspartate partially rescues tumour growth in patient-derived enteroids and CRC cell lines in the presence of an iron chelator. Collectively, these data suggest that ectopic hepcidin in the tumour epithelium establishes an axis to sequester iron in order to maintain the nucleotide pool and sustain proliferation in colorectal tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files. Source data are provided with this paper.
References
Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kroemer, G. & Pouyssegur, J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482 (2008).
Xue, X. & Shah, Y. M. Intestinal iron homeostasis and colon tumorigenesis. Nutrients 5, 2333–2351 (2013).
Drakesmith, H., Nemeth, E. & Ganz, T. Ironing out ferroportin. Cell Metab. 22, 777–787 (2015).
Brookes, M. J. et al. A role for iron in Wnt signalling. Oncogene 27, 966–975 (2008).
Le, N. T. & Richardson, D. R. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Biophys. Acta 1603, 31–46 (2002).
Xue, X. et al. Iron uptake via DMT1 integrates cell cycle with JAK–STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 24, 447–61 (2016).
Xue, X. et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72, 2285–2293 (2012).
Brookes, M. J. et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 55, 1449–1460 (2006).
Dixon, S. J. & Stockwell, B. R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Shen, J. et al. Iron metabolism regulates p53 signaling through direct heme–p53 interaction and modulation of p53 localization, stability and function. Cell Rep. 7, 180–193 (2014).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Bessman, N. J. et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science 368, 186–189 (2020).
Lakhal-Littleton, S. et al. Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc. Natl Acad. Sci. USA 116, 13122–13130 (2019).
Lakhal-Littleton, S. et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. eLife 5, e19804 (2016).
Malerba, M. et al. Epidermal hepcidin is required for neutrophil response to bacterial infection. J. Clin. Invest. 130, 329–34 (2020).
Pinnix, Z. K. et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2, 43ra56 (2010).
Xue, D., Zhou, C. X., Shi, Y. B., Lu, H. & He, X. Z. Decreased expression of ferroportin in prostate cancer. Oncol. Lett. 10, 913–916 (2015).
Hinoi, T. et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 67, 9721–9730 (2007).
Dame M. K., et al. Identification, isolation and characterization of human LGR5-positive colon adenoma cells. Development 145, dev153049 (2018).
Triner, D. & Shah, Y. M. Hypoxia-inducible factors: a central link between inflammation and cancer. J. Clin. Invest. 126, 3689–3698 (2016).
Xue, X., Ramakrishnan, S. K. & Shah, Y. M. Activation of HIF-1α does not increase intestinal tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G187–G195 (2014).
Andrews, N. C. Forging a field: the golden age of iron biology. Blood 112, 219–230 (2008).
Meyers, R. M. et al. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat. Genet. 49, 1779–84 (2017).
Ahn, C. S. & Metallo, C. M. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 3, 1 (2015).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Sullivan, L. B. et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 20, 782–788 (2018).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Zhang, S. et al. Disordered hepcidin–ferroportin signaling promotes breast cancer growth. Cell. Signal. 26, 2539–2550 (2014).
Tesfay, L. et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 75, 2254–2263 (2015).
Ward, D. G. et al. Increased hepcidin expression in colorectal carcinogenesis. World J. Gastroenterol. 14, 1339–1345 (2008).
Blanchette-Farra, N. et al. Contribution of three-dimensional architecture and tumor-associated fibroblasts to hepcidin regulation in breast cancer. Oncogene 37, 4013–4032 (2018).
Merle, U., Fein, E., Gehrke, S. G., Stremmel, W. & Kulaksiz, H. The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology 148, 2663–2668 (2007).
Liu, Q., Davidoff, O., Niss, K. & Haase, V. H. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J. Clin. Invest. 122, 4635–4644 (2012).
Villa, E., Ali, E. S., Sahu, U. & Ben-Sahra, I. Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers 11, 688 (2019).
Buj, R. & Aird, K. M. Deoxyribonucleotide triphosphate metabolism in cancer and metabolic disease. Front. Endocrinol. 9, 177 (2018).
Santana-Codina, N. et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat. Commun. 9, 4945 (2018).
Nurtjahja-Tjendraputra, E., Fu, D., Phang, J. M. & Richardson, D. R. Iron chelation regulates cyclin D1 expression via the proteasome: a link to iron deficiency-mediated growth suppression. Blood 109, 4045–4054 (2007).
Thelander, L., Graslund, A. & Thelander, M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem. Biophys. Res. Commun. 110, 859–865 (1983).
Khodour, Y., Kaguni, L. S. & Stiban, J. Iron–sulfur clusters in nucleic acid metabolism: varying roles of ancient cofactors. Enzymes 45, 225–56 (2019).
Triner, D. et al. Epithelial hypoxia-inducible factor-2α facilitates the progression of colon tumors through recruiting neutrophils. Mol. Cell. Biol. 37, e00481-16 (2017).
Solanki, S., Devenport, S. N., Ramakrishnan, S. K. & Shah, Y. M. Temporal induction of intestinal epithelial hypoxia-inducible factor-2α is sufficient to drive colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G98–G107 (2019).
Triner, D. et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology 156, 1467–1482 (2019).
Schwartz, A. J. et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Invest. 129, 336–348 (2019).
Dame, M. K. et al. Human colonic crypts in culture: segregation of immunochemical markers in normal versus adenoma derived. Lab. Invest. 94, 222–234 (2014).
Gyorffy, B., Surowiak, P., Budczies, J. & Lanczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 8, e82241 (2013).
Yuan, M., Breitkopf, S. B., Yang, X. & Asara, J. M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 (2012).
Subramanian, A. et al. Gene-set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Bennett, B. D., Yuan, J., Kimball, E. H. & Rabinowitz, J. D. Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat. Protoc. 3, 1299–1311 (2008).
Acknowledgements
We thank the laboratory of Y.M.S. and Zhaoqi Li for helpful input and suggestions. This work was supported by National Institutes of Health (NIH) grants R01CA148828, R01DK095201 and R01CA245546 (to Y.M.S.). C.A.L. was supported by the National Cancer Institute (R37CA237421, R01CA248160, R01CA244931 and R01CA215607). C.A.L. and Y.M.S. were supported by University of Michigan Rogel Cancer Center Core Grant (P30CA046592) and University of Michigan Center for Gastrointestinal Research (P30DK034933). Metabolomics studies performed at the University of Michigan were supported by NIH grant DK097153, the Charles Woodson Research Fund and the UM Pediatric Brain Tumor Initiative. X.X. was supported by the NIH (P20 GM130422 and K01DK114390), a Research Scholar Grant from the American Cancer Society (RSG-18-050-01-NEC), a Research Scholar Award from the American Gastroenterological Association and a Research Program Support Pilot Project Award from UNM Comprehensive Cancer Center (P30CA118100). P.P.H. (2T32CA071345-21A1), B.T.D. (T32GM007753), A.J.S. (F31DK116555), B.C. (T32GM007315) and S.A.K. (F31CA24745701) were supported by the indicated grants. Enteroid work was conducted with The Michigan Medicine Translational Tissue Modeling Laboratory, a funded initiative with support from the Endowment for Basic Sciences (Center for Gastrointestinal Research, Office of the Dean, Comprehensive Cancer Center, Departments of Pathology, Pharmacology and Internal Medicine).
Author information
Authors and Affiliations
Contributions
A.J.S. and Y.M.S. conceived and designed the study. A.J.S., J.W.G., S.S., S.A.K., B.C., C.C., P.P.H., B.T.D., R.S., M.K.D. and H.-J.L. acquired the data. A.J.S., J.W.G., S.S., S.A.K., B.C., C.C., P.P.H., B.T.D., R.S., M.K.D., H.-J.L., J.R.S., S.L.-L., M.G.V.H., C.A.L., X.X. and Y.M.S. developed the methodologies. A.J.S., J.W.G., S.S., S.A.K., B.C., C.C., P.P.H., B.T.D., R.S., M.K.D., H.-J.L., J.R.S., S.L.-L., M.G.V.H., C.A.L., X.X. and Y.M.S. analysed and interpreted the data. A.J.S. and Y.M.S. wrote the manuscript. Y.M.S. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
P.P.H. is a consultant for Auron Therapeutics. C.A.L. is an inventor on patents pertaining to Kras-regulated metabolic pathways, redox control pathways in pancreatic cancer and targeting GOT1 as a therapeutic approach (US patent nos: 2012112933-A1, 2015126580-A1 and 20190136238). All other authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Kevin Myant and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
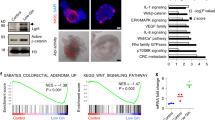
Extended Data Fig. 1 Hepcidin expression is increased across many cancer types.
a, Hepcidin transcript abundance in normal and tumor tissue through The Cancer Genome Atlas. Log2 median centered ratio is plotted (Bladder Normal N = 24 and Bladder Tumor N = 152; Brain Normal N = 10 and Brain Tumor N = 547; Colorectal Normal N = 22 and Colorectal Tumor N = 215; Breast Normal N = 61 and Brest Tumor N = 532; Cervical Normal N = 5 and Cervical Tumor N = 155; Head/Neck Normal N = 74 and Head/Neck Tumor N = 388; Kidney Normal N = 329 and Kidney Tumor N = 727; Leukemia Normal N = 195 and Leukemia Tumor N = 197; Liver Normal N = 30 Liver Tumor N = 138; Lung Normal N = 71 and Lung Tumor N = 984, Lymphoma Normal N = 18 and Lymphoma Tumor N = 18, Ovary Normal N = 88 and Ovary Tumor N = 607; Pancreas Normal N = 10 and Pancreas Tumor N = 64; and Prostate Normal N = 34 and Prostate Tumor N = 171 biologically independent samples, Brain p = 0.0047. Breast p = 0.0009. Colorectal p = 0.0006. Kidney p < 0.0001, Lung p = 0.0092. Ovary p = 0.0002. Prostate p = 0.0222). b, qPCR analysis of hepcidin (Hamp) expression levels in tissues/cells of wild-type and colon epithelial-specific APC deficient mice (WT N = 4 and APC-/- N = 4 biologically independent samples from independent animals in each tissue). c, Hepcidin concentration in serum of wild-type and colon epithelial-specific APC deficient mice (WT N = 4 and APC-/- N = 4 biologically independent samples from independent animals). Data represent the mean ± SEM. Significance was determined by 2-tailed, unpaired t test. a-c, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to normal tissue or wild-type animals.
Extended Data Fig. 2 Detection of the hepcidin protein by immunohistochemistry is ineffective.
Primary hepatocytes were generated from Hampfl/fl mice and treated in vitro with adenoviruses expressing either GFP or Cre recombinase for 48 hours. These cells were analyzed for hepcidin (Hamp) expression by qPCR analysis (a) and were stained for hepcidin protein (b) (AdenoGFP N = 3 and AdenoCre N = 3 biologically independent cell replicates, p = 0.0025). c, Representative hepcidin staining in mice that were placed on either an iron replete (350 PPM) or low iron (< 5 PPM) for seven days (N = 4 biologically independent samples from independent animals). d, Ferroportin staining of adjacent normal colon and tumor tissue in a sporadic model of CRC in wild-type and hepcidin-deficient mice (N = 3 biologically independent samples from independent animals). Data represent the mean ± SEM. Significance was determined by 2-tailed, unpaired t test (a). **P < 0.01.
Extended Data Fig. 3 Tumoral hepcidin regulation in vitro and in vivo.
qPCR analysis for hepcidin (Hamp) after treatment with FG4592 (100 μM) or vehicle for 16 hours in (a) HCT116 cells (N = 3 biologically independent cell replicates) and (b) enteroids generated from mice with inducible, colon epithelial deletion of APC and p53 and activation of KRAS (N = 3 biologically independent samples). c, qPCR analysis of Hamp in the colon of mice with embryonic, intestinal epithelial-specific overexpression of HIF-2α (HIF-2αOE) compared to wild-type mice (HIF-2αWT) (HIF-2αWT N = 6 and HIF-2αOE N = 10 biologically independent samples from independent animals). d, qPCR analysis of Hamp in the colon of colon epithelial-specific HIF-2αWT and HIF-2αOE mice that are also deficient for APC for 30 days HIF-2αWT N = 3 and HIF-2αOE N = 3 biologically independent samples from independent animals). e, qPCR analysis of Hamp in HCT116 cells treated for 24 hours with conditioned media (CM) from RAW 264.7 macrophages that had been treated with 10 ng/mL LPS for 16 hours (N = 3 biologically independent cell replicates). f, Relative luciferase activity of the human hepcidin promoter in HCT116 cells treated with vehicle (Veh), live bacteria (Live), or heat-killed bacteria (HK), or (g) vehicle (Veh) or bacteria-derived metabolites (N = 3 independent cell replicates). h, Methylation status of the human hepcidin promoter in human colorectal cancer tissue (Normal N = 37 and Tumor N = 313). i, HCT116 cells treated with vehicle (Veh) or 5AZA (10 μM) for 72 hours and then treated with vehicle (Veh) and/or FG4592 (100 μM) for 16 hours and analyzed via Western blot analysis for DNA methyltransferase 1 (DNMT1) and (j) qPCR analysis for HAMP expression (N = 3 biologically independent cell replicates). k, HCT116 cells pretreated with or without FG4592 (100 μM) for 16 hours and then administered a panel of epigenetic modifiers for 24 hours. qPCR was used to measure the expression of HAMP and the HIF-2α target, ANKRD37 (N = 3 biologically independent cell replicates for each treatment). l, Patient-derived enteroids were either pretreated with or without FG4592 (100 μM) for 16 hours and were then administered a panel of epigenetic modifiers for 24 hours. qPCR was used to measure the expression of HAMP and the HIF-2α target, ANKRD37 (N = 3 biologically independent samples for each treatment). Data represent the mean ± SEM. Significance was determined by 2-tailed, unpaired t test (a-e, h) or 1-way ANOVA with Tukey’s post hoc (f, j, k-l). ****P < 0.0001.
Extended Data Fig. 4 Colon cancer-derived cell lines are exquisitely sensitive to ferroportin-mediated iron loss.
a, qPCR analysis for the ferroportin transcript in late passages of cells that were made stable for a doxycycline (dox) inducible ferroportinGFP overexpression construct, following 16 hour treatment with dox (250 ng/mL) (N = 3 biologically independent cell replicates for each cell line and treatment, HEK293 p = 0.0001, IEC6 p = 0.0021, HT29 p < 0.0001, SW480 p = 0.0103) (b) Western blot analysis for GFP and ferroportin (FPN) in stable, normal ferroportinGFP overexpressing cell lines following 16 hour treatment with dox (250 ng/mL). c and d, Cell growth MTT assay in HEK293 ferroportinGFP (c) (N = 3 biologically independent cell replicates for each cell line, treatment, and time point, p < 0.0001) and (d) IEC6 ferroportinGFP cells (N = 3 biologically independent cell replicates for each cell line, treatment, and time point, p = 0.0008) treated with vehicle, dox (250 ng/mL), or dox (250 ng/mL) and recombinant hepcidin (1 μg/mL). e–h, Representative crystal violet staining images (e) and quantification (f) of HEK293 ferroportinGFP (N = 3 biologically independent cell replicates for each cell line and treatment, p = 0.0005) and IEC6 ferroportinGFP cells, images (g) and quantitation (h) (N = 3 biologically independent cell replicates for each cell line and treatment, p = 0.0025) ten days following treatment with vehicle, dox (250 ng/mL), or dox (250 ng/mL) and recombinant hepcidin (1 μg/mL) Data represent the mean ± SEM. Significance was determined by 2- tailed, unpaired t test (a) or by 1-way ANOVA with Tukey’s post hoc (c,d,f,h). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 comparing within each cell line or across treatment groups.
Extended Data Fig. 5 Intracellular iron deficiency blunts nucleotide metabolism.
a, Representative patient-derived CRC tumor enteroids were treated with vehicle or DFO (10 μM) (N = 3 biologically independent replicates). b, Quantification of enteroid growth following 4-days of either vehicle or DFO (10 μM) treatment (N = 3 biologically independent replicates). c, Pathway analysis was conducted on metabolomics data in enteroids after DFO treatment before any decrease in growth was seen. Significance was determined by 2-tailed, unpaired t test.
Extended Data Fig. 6 Intracellular iron deficiency blunts nucleotide metabolism.
a,b, Heatmap of metabolites of differential abundance in (a) HEK293 and (b) IEC6 doxycycline- inducible ferroportin overexpressing cell lines treated with doxycycline (d) as compared to vehicle (V) for 16 hours (N = 3 biologically independent cell replicates, only changes at p < 0.05 compared to V are shown). c, Pie chart showing the proportion of metabolites of differential abundance in both HEK293 and IEC6 ferroportin overexpressing cells that are involved in nucleotide metabolism.
Extended Data Fig. 7 Moderate doses of DFO do not remove iron from ferritin and individual nucleosides do not rescue DFO-mediated growth inhibition.
a and b, Western blot analysis for ferritin after 24 hours pre-loading with ferric ammonium citrate (FAC) (200 μM) and/or overnight DFO, as indicated in (a) HCT116 and (b) SW480 cells. c and d, Relative growth of HCT116 cells as measured by MTT at 72 hours after treating with DFO (10 μM) and individual nucleosides (100 μM) (c) (Vehicle N = 6, DFO N = 6, DFO + Nucloeside N = 8 biologically independent cell replicates, p < 0.0001), or (d) purine or pyrimidine (100 μM) nucleosides (N = 8 biologically independent cell replicates for all treatments, p < 0.0001. e, Cell growth MTT assay in IEC6 ferroportinGFP cells treated with vehicle, dox (250 ng/mL), or dox (250 ng/mL) and nucleoside cocktail (100 μM) 72 hours after treatment (Vehicle N = 6, Dox N = 9, Nuc N = 9, and Dox + Nuc N = 9 biologically independent cell replicates, p = 0.0006). f and g, Nucleoside (100 μM) rescue of DFO (10 μM) growth inhibition in DLD1 (f) (N = 8 biologically independent cell replicates for all treatments, p = 0.0007) and RKO (g) (N = 8 biologically independent cell replicates for all treatments, p = 0.0018 cells) after 72 hours of cotreatment using MTT. h and i, Representative crystal violet stains of DLD1 or RKO cells that were either pretreated with vehicle or DFO (10 μM) and then given vehicle or a nucleoside cocktail (100 μM) for ten days (N = 3 biologically independent cell replicates). Data represent the mean ± SEM. Significance was determined by one-way ANOVA followed by Tukey’s post hoc. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to vehicle.
Extended Data Fig. 8 Acute DFO insult disrupts mitochondrial metabolism without decreased cell growth.
a, Cell growth assay in HCT116 and SW480 cells 24 hours after DFO treatment (10μM) and assessed by MTT (N = 3 biologically independent cell replicates for each treatment and cell line). b and c, Seahorse analysis of mitochondrial metabolism in DLD1 (b) and RKO (c) cells 24 hours after DFO administration (10μM) (N = 4 biologically independent cell replicates for each treatment condition). d and e, Western blot analysis of doxycycline (Dox)-inducible Lactobacillus NADH-oxidase (LbNox) and mitochondrial NADH-oxidase (Mito-LbNox) in stably generated in HCT116 (d) and SW480 (e) cells. f and g, MTT cell growth assays in LbNox and Mito-LbNox expressing cells following DFO (10μM) in (f) HCT116 (p = 0.014) and (g) SW480 cells (p = 0.0076) (N = 6 biologically independent cell replicates for each cell line and treatment). h and i, MTT cell growth assay in cells stably expressing yeast NADH-ubiquinone reductase (NDI1) following DFO (10μM) or phenformin (62.5μM) treatment in (h) HCT116 (p = 0.0006) and (i) SW480 (p = 0.0003) cells (N = 3 biologically independent cell replicates for each cell line and treatment). j and k, Western blot analysis of mitochondrial enzymes following 24-hours of DFO (10 µM) treatment in DLD1 (j) and RKO (k) cells. Data represent the mean ± SEM. Significance was determined by 2-tailed, unpaired t test (a, h, i)) or one-way ANOVA (f–g) followed by Tukey’s post hoc. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to vehicle.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8
Supplementary Table 1
DepMap data analysis.
Supplementary Table 2
Metabolomics dataset.
Supplementary Table 3
Primer sequences.
Source data
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Schwartz, A.J., Goyert, J.W., Solanki, S. et al. Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat Metab 3, 969–982 (2021). https://doi.org/10.1038/s42255-021-00406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00406-7
This article is cited by
-
Linc00707 regulates autophagy and promotes the progression of triple negative breast cancer by activation of PI3K/AKT/mTOR pathway
Cell Death Discovery (2024)
-
Advancing the frontiers of colorectal cancer treatment: harnessing ferroptosis regulation
Apoptosis (2024)
-
β-catenin-IRP2-primed iron availability to mitochondrial metabolism is druggable for active β-catenin-mediated cancer
Journal of Translational Medicine (2023)
-
Synergistic immunotherapy targeting cancer-associated anemia: prospects of a combination strategy
Cell Communication and Signaling (2023)
-
USP11-mediated LSH deubiquitination inhibits ferroptosis in colorectal cancer through epigenetic activation of CYP24A1
Cell Death & Disease (2023)