Mitigation of AFB1-Related Toxic Damage to the Intestinal Epithelium in Broiler Chickens Consumed a Yeast Cell Wall Fraction
- 1Unidad de Investigación Multidisciplinaria L14 (Alimentos, Micotoxinas, y Micotoxicosis), Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Mexico City, Mexico
- 2Departamento de Medicina y Zootecnia de Aves, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de Mexico, Mexico City, Mexico
- 3Department of Poultry Science, University of Arkansas, Fayetteville, AR, United States
In vivo experiments were conducted to evaluate the effectiveness of a yeast cell wall fraction (YCW) to reduce the negative impact of aflatoxin B1 (AFB1) to the intestinal epithelium in broiler chickens. Zeta potential (ζ-potential), point of zero charge (pHpzc), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM) techniques were used to characterize the YCW. Two hundred one-day-old male Ross 308 broiler chickens were randomly allocated into four treatments: (1) control, chickens fed an AFB1-free diet; (2) AF, chickens feed an AFB1-contaminated diet (500 ng AFB1/g); (3) YCW, chickens fed an AFB1-free diet + 0.05% YCW; and (4) AF + YCW, chickens fed an AFB1-contaminated diet (500 ng AFB1/g) + 0.05% YCW. At the end of the 21-day feeding period, fluorescein isothiocyanate dextran (FITC-d) was administered to chicks by oral gavage to evaluate gastrointestinal leakage. Blood and duodenum samples were collected to assess serum biochemistry and histomorphology, respectively. Compared to the control group, chicks of the AF group significantly diminished weight gain (WG) and average daily feed intake (ADFI), and increased feed conversion ratio (FCR), mortality rate (MR), and intestinal lesion scores (p < 0.05). Alterations in some serum biochemical parameters, and damage to the intestinal integrity were also evident in the AF-intoxicated birds. YCW supplementation improved WG and FCR and increased villus height, villus area, crypt depth, and the number of goblet cells in villi. The effects of YCW on growth performance were not significant in chicks of the AF + YCW group; however, the treatment decreased MR and significantly ameliorated some biochemical and histomorphological alterations. The beneficial effect of YCW was more evident in promoting gut health since chickens of the AF + YCW group presented a significant reduction in serum FITC-d concentration. This positive effect was mainly related to the changes in negative charges of YCW due to changes in pH, the net negative surface charge above the pHpzc, the higher quantities of negative charged functional groups on the YCW surface, and its ability to form large aggregates. From these results, it can be concluded that YCW at low supplementation level can partially protect broilers' intestinal health from chronic exposure to AFB1.
Introduction
Filamentous fungi, particularly Aspergillus, Fusarium, and Penicillium, are capable of forming secondary metabolites known as mycotoxins. Among the many toxic metabolites identified, some of them are potent carcinogens, which can provoke acute or chronic intoxications in both humans and animals. In agricultural commodities, the most frequently encountered mycotoxins are aflatoxins, ochratoxin A, patulin, fumonisins, trichothecenes (deoxynivalenol, T-2 toxin, and HT-2), and zearalenone (1). Some mycotoxigenic fungi can produce more than one toxin, and some mycotoxins are synthesized by multiple fungal species (2). In comparison with other mycotoxins, the safety level for aflatoxins in poultry feedstuffs is low; as a result, poultry feed is always at risk of contamination with aflatoxins, which are frequently found in maize destined for animal feed. When toxigenic Aspergillus flavus, Aspergillus parasiticus, or Aspergillus nomius isolates grow in poultry feedstuffs, they can synthesize a variety of toxic secondary metabolites, including aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2). As a result, the accumulation of these toxic metabolites in animal tissues may result in an indirect exposure to humans by consuming the contaminated products such as meat or eggs.
Notwithstanding attempts to monitor fungal and mycotoxin contamination, both developing and developed countries have confirmed widespread contamination. To detoxify mycotoxin-contaminated feeds and feedstuffs, various methods have been proposed based on physical, chemical, and biological approaches. Detoxifying agents are substances that may reduce mycotoxin contamination in feed by suppressing or reducing their absorption, promoting their excretion, or changing their mode of action. These substances—called mycotoxin detoxifiers—are added to animals' diet (poultry, swine, and cattle) to minimize toxin absorption and dissemination to blood and target organs. Based on their mode of action, mycotoxin detoxifiers can bind, inactivate, degrade, or transform mycotoxins into less toxic substances. Activated charcoal, hydrated sodium calcium aluminosilicates, polymers, zeolites, agro-waste materials, yeast, and yeast products are examples of adsorbent materials that can be utilized to reduce the toxic effects of various mycotoxins (2–4).
Yeast cell wall (YCW), mainly composed of polysaccharides (mannans and glucans), proteins, and lipids, possesses a variety of adsorption sites, with different mechanisms of adsorption including hydrogen bonding, hydrophobic interactions, and ionic interactions (5). Therefore, YCW could be an alternative over conventional adsorbent materials to bind a wide variety of mycotoxins (6), without reducing nutrient bioavailability or causing negative environmental impacts. While many studies have been conducted to show that YCW can improve broiler performance and intestinal health when challenged with aflatoxins alone or in combination with pathogens (7, 8), just a few studies have looked into the pathways that lead to the formation of complexes involving mycotoxins and YCW components, where some chemical structures such as (1 → 3)-β-D-glucan or (1 → 6)-β-D-glucan play a significant role during the binding process (9–11). Currently, there is a lack of general knowledge about the application of ζ-potential, point of zero charge, Fourier transform infrared spectroscopy, and scanning electron microscopy techniques to characterize the YWC and to understand the interaction between the functional groups present on the YCW surface and the AFB1 molecule. Consequently, this research aimed to describe and evaluate the effectiveness of a commercial YCW fraction's low content to reduce the negative impact of AFB1 on the intestinal epithelium in broiler chickens.
Materials and Methods
Yeast Cell Walls
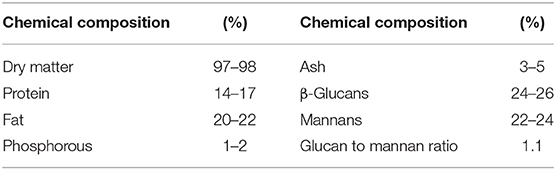
A premium yeast cell wall fraction (YCW) from Saccharomyces cerevisiae (SafMannan) was kindly provided by Phileo Lesaffre Animal Care (Lesaffre Iberica S.A., Valladolid, Spain). The chemical composition of the commercial YCW fraction according to the manufacturer is shown in Table 1.
YCW Characterizations
Zeta Potential (ζ-Potential)
The electrophoretic mobility measurement and conversion to ζ-potential were made using the ZetaSizer Pro (Malvern Instruments, Worcestershire, UK) following the methodology of Ramales-Valderrama et al. (12). All determinations were done at room temperature by diluting 500 μL of the YCW suspension (0.05% w/v) in 5 ml deionized water. Quintuplicates were evaluated, and each measurement included 30 runs to find a stable reading. Samples were evaluated at three different pH values simulating the poultry gastrointestinal tract's in vivo conditions (proventriculus, pH 2; crop, pH 5; and intestine, pH 7).
Point of Zero Charge (pHpzc)
The pHpzc was determined following the approach used by Zavala-Franco et al. (13). Briefly, 50 mL of distilled water was adjusted to different pH values (2, 4, 6, 8, 10, and 12) by adding 0.1 M hydrochloric acid or 0.1 M sodium hydroxide. The solutions were added into flasks containing preweighed YCW (25 mg) and stirred (250 rpm) at room temperature for 195 min. The final pH (pHf) of the suspension was determined, and the pH difference (ΔpH) was computed. All pH measurements were accomplished using a glass electrode (Conductronic PC-45, Puebla, Mexico). Finally, ΔpH was plotted against the initial pH (pHi), and the point where the line intersects the x-axis gave the pHpzc. All determinations were performed in quintuplicate.
Fourier Transform Infrared Spectroscopy
Functional groups of the YCW were characterized using a Fourier transform infrared spectroscopy (FTIR) Frontier SP8000 spectrophotometer (Perkin Elmer, Waltham, MA, USA) accessorized with an attenuated total reflection (ATR) accessory (DuraSamplIR II, Smiths Detection, Warrington, UK). Quintuplicate samples (25 mg) were placed on the ATR diamond crystal, and the spectra were recorded in transmittance mode over the range of 4,000–500 cm−1 at a resolution of 4 cm−1 by coadding 32 scans. The background spectrum of air was subtracted from all the spectra. Additionally, a section of the spectra (the polysaccharide absorbing region, which reveals component structures of mannans and glucans) was baseline corrected, and the resultant FTIR spectrum was further analyzed. The peak areas of the main bands (carbohydrate, protein, and lipid) were computed using the Spectrum 10.4.2 software.
Scanning Electron Microscopy
The morphology and microstructure of the YCW were scrutinized using an InTouch Scope scanning electron microscope (SEM) (JEOL, JSM-6012LA, Tokyo, Japan). To enhance electron conductivity and image quality, samples were coated with a thin gold layer using an electric sputter coater (Denton Vacuum Inc., Desk V HP, Moorestown, NJ, USA) operated at 7 mA for 3 min. Microscopy analysis (×250) was performed in the secondary electron imaging mode (SEI mode) with an accelerating voltage of 15 kV at a 17-mm effective working distance.
In vivo Experiments
Animal Ethics
The aflatoxin challenge protocol was approved by the Internal Committee for Care and Use of Experimental Animals of the Postgraduate Program in Animal Production and Health Sciences of the National Autonomous University of Mexico. Ethical approval code: CICUAE-C20_5.
Aflatoxin B1 Production and Preparation of the AFB1-Contaminated Diet
Aflatoxins (AFB1 and AFB2) were produced in maize according to the methodology of Méndez-Albores et al. (14) using a highly toxigenic strain of A. flavus (Code UNIGRAS-1231, Culture Collection of the Grain and Seed Research Unit of the National Autonomous University of Mexico). The highly contaminated maize kernels (14,000 ng AFB1/g) were milled and subsequently mixed in a starter feed formulated to approximate broiler chickens' nutritional requirements (Supplementary Table 1) as recommended by the National Research Council (15). Contamination was performed in batches of 15 kg using 36 g of the aflatoxin-contaminated maize meal per kilogram of feed. Subsequently, the aflatoxin-contaminated poultry feed was mixed for 15 min in a Ribbon Blender Mixer (Molinos Pulvex model MH-7050, Mexico City, Mexico) to ensure proper distribution of the toxins. The adsorbent material was also included in the feed.
Aflatoxin B1 Quantification
The aflatoxin content in the feed was estimated by immunoaffinity column clean-up and liquid chromatography with fluorescence detection. Briefly, aflatoxins were cleaned up using immunoaffinity columns Vicam Afla B (Watertown, MA, USA) and the eluate used for ultraperformance liquid chromatography (UPLC) analysis. A modified method previously described by Jardon-Xicotencatl et al. (16) was used. A mobile phase of water/methanol/acetonitrile (64:18:18) was used on an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm). The mobile phase was pumped at 0.7 mL/min by a quaternary solvent manager. The aflatoxins eluted in the order of AFB2 and AFB1 at 1.57 and 2.00 min, respectively (Supplementary Figure 1). Detection was via an UPLC-optimized fluorescence detector (Waters, Milford, MA, USA) programmed to detect aflatoxins at 365 nm excitation and 429 nm emission. The estimated detection limits were 0.6 and 2.0 ng/kg for AFB2 and AFB1, respectively. Finally, the AFB1 concentration was calculated using a standard reference (AFB1; CAS number, 1162-65-8, Merck KGaA, Darmstadt, Germany) with a calibration curve. All determinations were done in quintuplicate. The UPLC analysis revealed the presence of AFB1 (500 ± 21 ng/g feed) and traces of AFB2 (43 ± 7 ng/g feed). Since AFB2 is up to 200-fold less toxic than AFB1 (14), in this research, the presence of AFB2 was considered insignificant.
Experimental Birds and Housing
A total of 200 1-day-old male broilers (Ross 308) were purchased from a local hatchery. Birds were randomly distributed in four pens at the Poultry Research Station of the National Autonomous University of Mexico. Five replicates of 10 birds (n = 50 per treatment) were grouped as follows: (1) control, chickens fed an AFB1-free diet; (2) AF, chickens feed an AFB1-contaminated diet (500 ng AFB1/g); (3) YCW, chickens fed an AFB1-free diet + 0.05% YCW (the minimum manufacturers' recommended inclusion rate); and (4) AF + YCW, chickens feed an AFB1-contaminated diet (500 ng AFB1/g) + 0.05% YCW. Chicks were maintained at an age-appropriate temperature and given ad libitum access to diets and water during the 21-day study. Twice a day, birds were monitored for general health.
Collection of Samples and Measurements
Birds and feed were weighed weekly, and feed efficiency was adjusted for mortality. At 21 days of age, blood samples were collected from 15 randomly selected broilers from each treatment (three birds per replicate) and serum prepared. The following analyses were accomplished spectrophotometrically using commercially available kits (BioSystems, Barcelona, Spain): total protein (code 11500), albumin (code 11547), glucose (code 12503), cholesterol (code 11505), and the enzymatic activities of aspartate aminotransferase (AST, code 11531) and alanine aminotransferase (ALT, code 21533). The bled chickens were then euthanized by CO2 inhalation, and segments of duodenum (2 cm in length) taken from the gizzard outlet to the end of the pancreatic loop were carefully excised, rinsed three times with cold saline, and fixed in 10% neutral-buffered formalin for 48 h. The paraffin-embedded tissue samples were cut into 4-μm thick sections and stained with hematoxylin and eosin (H&E). Photomicrographs were acquired using an ICC50W camera associated with a Leica DM2500 microscope. The variables measured were the following: villus height (measured from the top of the villus to the upper part of the lamina propria), villus width (taken at the central part of the villus), crypt depth (measured from the base up to the region of transition between the crypt and villus), villus area (villus height × villus width), and the goblet cell number along the villi membrane, which were counted along 500 μm of each villus surface. The ImageJ 1.52v software was used for morphometric measurements. In each treatment, 60 measurements were taken per variable.
Serum Determination of Fluorescein Isothiocyanate Dextran Leakage
Fluorescein isothiocyanate dextran (3–5 kDa, Merck KGaA, Darmstadt, Germany) was used as a probe to measure gut mucosal barrier integrity. Following the methodology of Baxter et al. (17), 1 h before euthanizing the chickens, 15 randomly selected broilers of each group (three per replicate) were orally gavaged with fluorescein isothiocyanate dextran (FITC-d) (8.32 mg/kg of body weight). The concentration of FITC-d was fluorometrically estimated in diluted sera as described by Hernández-Ramírez et al. (18). Sera from birds without FTIC-d treatment were used as controls.
Experimental Design and Statistical Analysis
Data were analyzed as a completely randomized design using the one-way ANOVA procedure of the Statistical Analysis System software (19). The replicate pens were the experimental units for the analysis, and means were separated using the Tukey procedure at p < 0.05 level of significance.
Results
Characterizations of the YCW Fraction
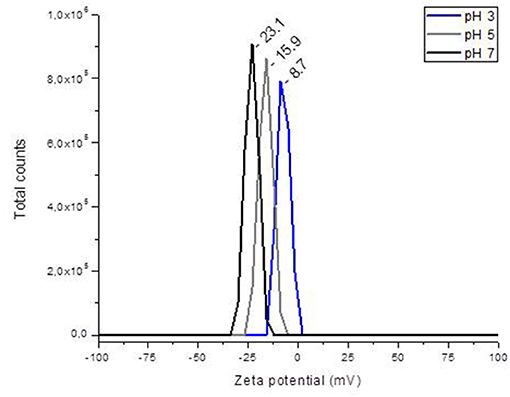
Figure 1 shows the relationship between ζ-potential and pH. In general, the YCW product had a negative ζ-potential; however, as the pH value increased (from 2 to 7), more negative ζ-potential values were observed. A ζ-potential value of −8.7 ± 1.3 mV was registered at pH 2; however, at pH 5 and 7, the YCW preparation presented ζ-potential values of −15.9 ± 2.5 mV and −23.1 ± 2.1 mV, respectively. The point of zero charge (pHpzc) was determined by plotting ΔpH against the initial pH using the immersion technique. Figure 2 shows the pHpzc of the YCW preparation. In the pHpzc graphic, the curve intersects the x-axis at pH 3.09, suggesting that the surface charge is zero at this particular pH. In other words, the charge of the positive surface sites is equal to that of the negative ones.
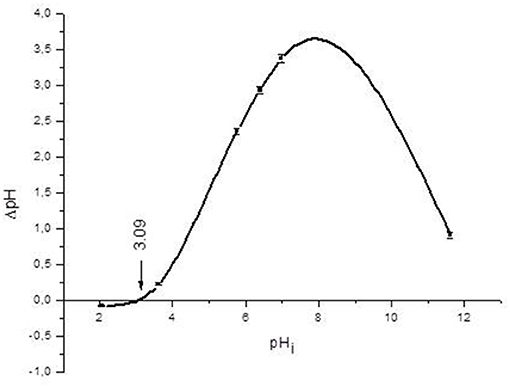
Figure 2. Point of zero charge (pHpzc) of the yeast cell wall fraction (YCW). Mean values ± standard error.
Furthermore, the FTIR spectrum was acquired to identify the specific functional groups present on the YCW product's surface. The representative FTIR spectrum and the baseline-corrected spectrum of the polysaccharide absorbing region (1,200–750 cm−1) are shown in Figure 3. From the spectrum, three main regions corresponding to polysaccharides (R1 = 750–1,200 cm−1), proteins (R2 = 1,400–1,650 cm−1), and lipids (R3 = 2,800–3,000 cm−1) can be clearly distinguished (Figure 3, profile A). Main absorptions characteristic of polysaccharides are those related to O–H stretching (3,279 cm−1), β-anomeric carbons of β-glucans (1,369 cm−1), C–O stretching and C–OH wagging (1,202 and 1,025 cm−1), β-anomeric carbons of β(1 → 3) glucans (887 cm−1), and mannans (810 cm−1). Characteristic N–H vibrations of proteins were observed at 3,279 cm−1 (overlapped by O–H vibrations) and at 1,629 and 1,532 cm−1, which were associated with the amide I and amide II bands, respectively. Finally, C–H stretching bands of lipids were located at approximately 2,922 and 2,849 cm−1, respectively. These bands were also overlapped by the C–H stretching of glucans (Figure 3, profile A). Moreover, Figure 3 (profile B) depicts the baseline-corrected FTIR spectrum of the carbohydrate absorbing region (frequency range, 1,200–750 cm−1). As can be seen from the spectral magnification, the absorptions at 810, 919, 970, and 1,052 cm−1 characterize mannans. Additionally, the bands at 887, 1,080, 1,107, and 1,151 cm−1 can be assigned to β(1 → 3) glucans. Finally, the broad absorption band at 1,025 cm−1 is generally associated with the presence of β(1 → 4) glucans. The assignments of the main vibrational bands are summarized in Table 2. Furthermore, as a useful indicator of the three main components' ratio, the total area corresponding to polysaccharide, protein, and lipid regions was computed using the Spectrum 10.4.2 software. The results show the highest intensity in the polysaccharide region, followed by lipid and protein regions, respectively. In general, the polysaccharide was shown approximately 3.9-fold higher than lipid, and the ratio of polysaccharide to protein was 7.8-fold higher. These results are consistent with the chemical composition of the YCW product shown in Table 1.
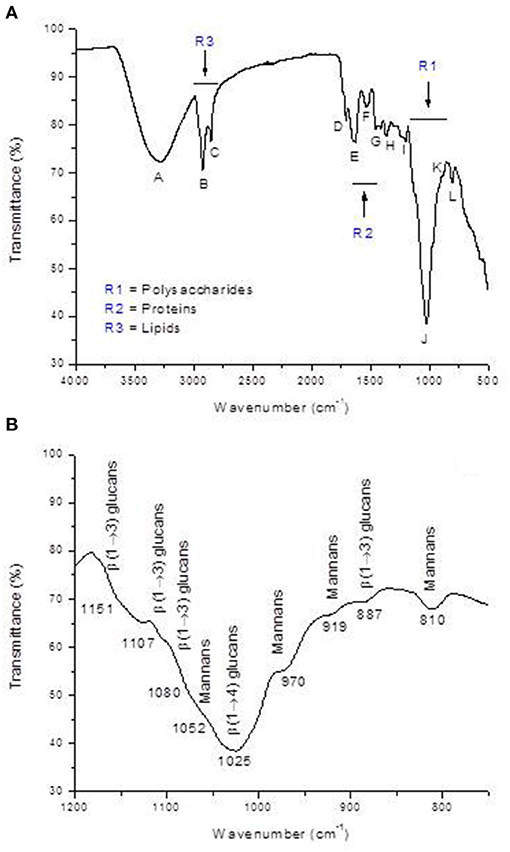
Figure 3. (A) Representative FTIR spectrum of the yeast cell wall fraction (YCW), and (B) baseline corrected FTIR spectrum of the polysaccharide absorbing region (R1 = 1200-750 cm−1).
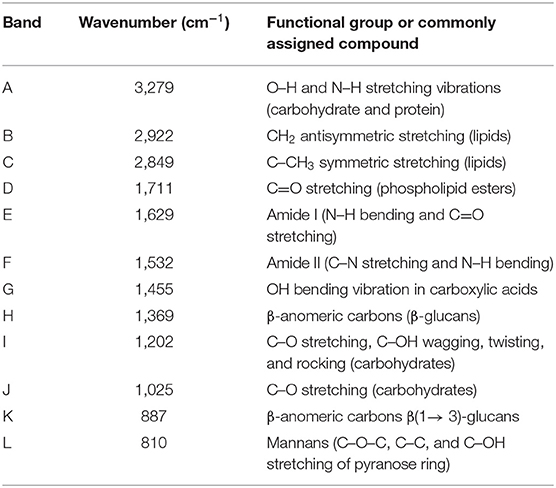
Table 2. Band assignments of the primary vibrational frequencies in the yeast cell wall fraction (YCW).
The surface morphology and microstructure of the YCW preparation were assessed using SEM. An illustrative micrograph is shown in Figure 4. The image shows mostly β-glucan particles ranging from 20 to 169 μm in size, with some single particles. The majority of the unaggregated particles was approximately 27 ± 3 μm in size; however, the formation of aggregates between β-glucan particles was more noticeable. The SEM image also reveals the ridge-like nature of the β-glucan, with smooth surfaces and rolled-up edges. Finally, the microstructure of β-glucan particles was retained as indicated by their distinctive oval shape.
In vivo Experiment
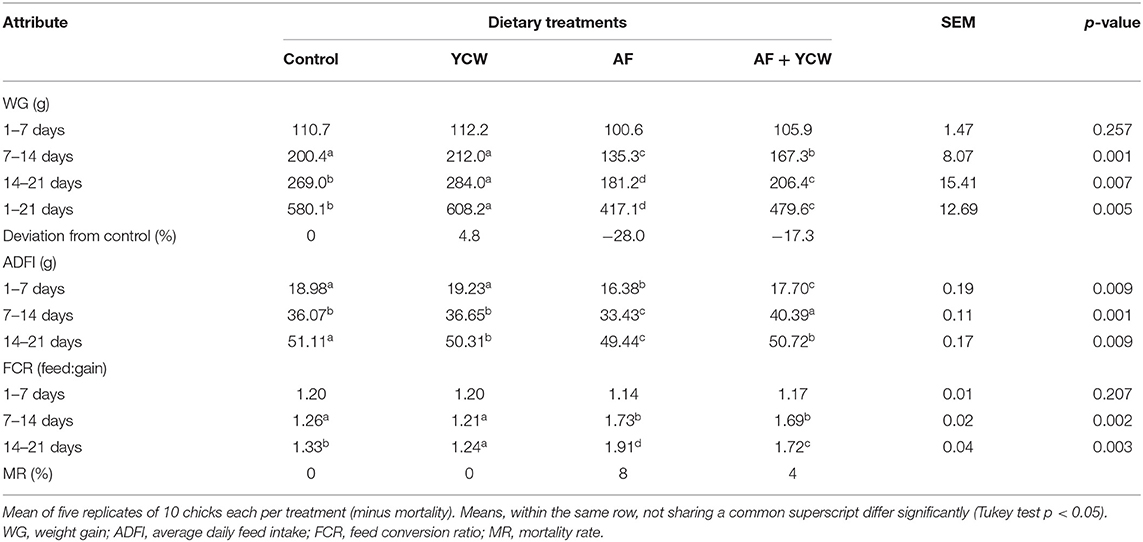
Data on the performance of experimental broilers are summarized in Table 3. At the end of week 1, no significant differences were noted in weight gain (WG) among the four dietary treatments. Nevertheless, by the end of week 2, WG was significantly reduced (p < 0.05) in chickens of the AF and AF + YCW groups, respectively. At the end of the trial (week 3), chickens receiving the AFB1-contaminated diet have a 28% reduction in WG. Moreover, WG was significantly improved (4.8%) in birds of the YCW treatment. Average daily feed intake (ADFI) was affected until 21 days of age. Furthermore, by the end of weeks 2 and 3, feed conversion ratio (FCR) was significantly affected in the AF and AF + YCW groups. Finally, the survival rate was as follows: 92% in the AF group, 96% in the AF + YCW group, and 100% in the control and YCW groups, respectively (Table 3). In general, the adverse effects in WG, ADFI, FCR, and survival rate—caused by AFB1–were significantly alleviated by the YCW treatment, which means that the YCW fraction offers reasonable protection against the harmful effects caused by AFB1.
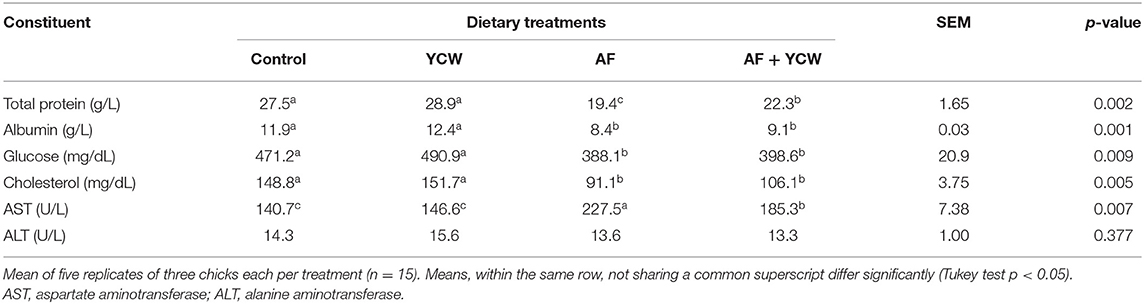
Table 4 summarizes the serum biochemical results. AFB1 caused a significant decrement in total protein, albumin, glucose, and cholesterol concentrations. Compared to the control group, reductions of 29.5, 29.4, 17.6, and 38.8% in those constituents were observed in chickens of the AF group. Additionally, some indications of AFB1 toxicity were distinguished in the AF and AF + YCW groups' chickens by the serum AST activity level, which increased by 1.6- and 1.3-fold in comparison with the control group, respectively. On the contrary, no significant differences were observed in the ALT activity among all dietary treatments (Table 4). In general, the YCW preparation alleviates most of the biochemical parameters in the serum altered negatively due to AFB1.
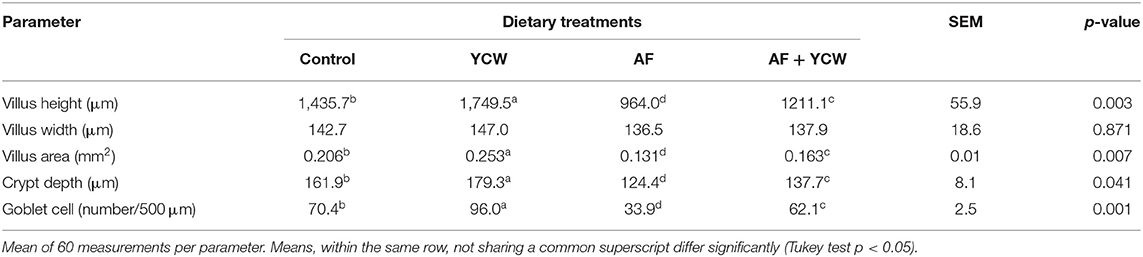
Histomorphological parameters of the broiler's duodenum are summarized in Table 5. In general, villus height, villus area, crypt depth, and the number of goblet cells were significantly lower in chickens fed the AFB1-contaminated diet. The addition of the YCW preparation to the AFB1-contaminated diet increased villus height (1,211.1 vs. 964.0 μm); however, the value was significantly lower than the observed in the control group (1,435.7 μm). Furthermore, compared to the control group, chickens of the YCW group have higher villus height, villus area, crypt depth, and the number of goblet cells in villi. Villus width was not affected by any dietary treatment (Table 5 and Supplementary Figure 2).
Data on serum concentrations of FITC-d are shown in Figure 5. No significant differences were noted in serum levels of FITC-d between the control and YCW groups. However, a significant increment in serum levels of FITC-d was detected in birds fed the AFB1-contaminated diet, reaching values up to 0.42 ± 0.05 μg FITC-d/ml serum. Interestingly, YCW supplementation of the AFB1-contaminated diet significantly diminished the serum levels of FITC-d (40.5%). The AFB1-related toxic damage to the intestinal epithelium in broiler chickens was partially mitigated by incorporating the YCW product into the diet.
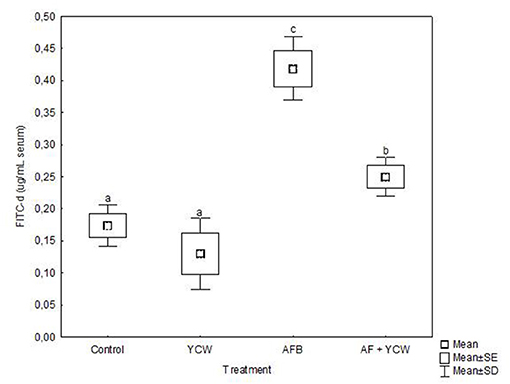
Figure 5. Serum fluorescein isothiocyanate dextran (FITC-d) levels in broiler chickens at 21 days of age. Mean of five replicates of three chicks each per treatment (n = 15). Boxes and whiskers not sharing a common superscript differ significantly (Tukey test P < 0.05).
Discussion
To confirm the effectiveness of the YCW preparation to bind AFB1, ζ-potential, pHpzc, FTIR, and SEM techniques were employed. The ζ-potential is a measurement of the charges on the surface of colloidal particles. Without a doubt, interface properties are significantly influenced by changes in pH, ionic strength, temperature, composition of the medium, among others. As a result, in this work, the YCW preparation was evaluated regarding its ζ-potential at pH values of the proventriculus (pH 2), crop (pH 5), and intestine (pH 7). It was observed that ζ-potential increased significantly with increasing pH reaching the maximum at pH 7 (Figure 1). This ζ-potential shift may be attributed to changes in cell wall charges. These results are in close agreement with Lavaisse et al. (20), who reported ζ-potential values of −6 and −16 mV for Saccharomyces cerevisiae cell wall at pH 3.5 and 5, respectively. On the other hand, the pHpzc also provides useful information about particles' surface charge. The results indicate that the surface charge of the YCW product was zero at pH 3.09 (Figure 2). Thus, the YCW surface remained negatively charged when pH > pHpzc > 3.09. As a result, the YCW preparation possesses significant AFB1-sorption uptakes in the crop (pH 5) and intestine (pH 7). On the contrary, in the proventriculus (pH 2), the contribution of electrostatic interactions would be drastically reduced, as the surface net charge of the YCW product is positive. In the present study, the high negative-charged surface of YCW particles (which remained mostly unchanged at pH values above the pHpzc) can be associated with their ability to remove AFB1 in some gastrointestinal tract compartments because of the enhancement of attractive forces between the AFB1 molecule and the YCW surface.
Infrared spectroscopy gives information at the molecular level, allowing the investigation of surface functional groups. In this research, the YCW preparation was further characterized to obtain information about the nature of the interaction between the functional groups present on the YCW surface and the AFB1 molecule. The FTIR spectrum of the YCW product is shown in Figure 3, profile A. In general, the YCW fraction exhibited higher quantities of functional groups associated with polysaccharides (3,279, 1,369, 1,202, 1,025, 887, and 810 cm−1), lipids (2,922, 2,849, and 1,711 cm−1), and proteins (3,279, 1,629, and 1,532 cm−1). These three main components have many different negatively charged functional groups responsible for the AFB1 adsorption (4, 10, 11). It has been reported that ~80% of the dry weight of the YCW product is made up of β-glucans and α-mannans (21). Besides, the outer layer of the S. cerevisiae cell wall is also composed of phosphomannans, which possess a net negative charge due to the presence of the phosphate group. However, the band associated with this functional group (located at approximately 1,070 cm−1) also appears in the polysaccharide absorbing region (1,200–750 cm−1); as a result, an in-depth band assignment is often complicated. However, glucan and mannan content in the YCW preparation was much higher than that of phosphates (Figure 3, profile B). These results corresponded well with transmittance intensities (or calculated areas) in the FTIR spectrum (Figure 3, profile A), showing the highest intensity/area in the polysaccharide region (R1), followed by lipid (R3) and protein (R2) regions, respectively.
The morphology and microstructure of the YCW product were examined by SEM. This material was determined to be a heterogeneous mixture of individual particles (27 ± 3 μm in size) and glucan particle aggregates up to 169 μm in length (Figure 4). Aggregation is a process in which materials joined together to generate themselves a mass or cluster, increasing or decreasing its porosity or density (12). In this research, the changes in cell wall negative charges due to changes in pH, the net negative surface charge above the pHpzc 3.09, the higher quantities of negative charged functional groups on the YCW surface, and the formation of aggregates improved the efficiency and functionality of the YCW preparation resulting in a material with significant interaction with AFB1.
Aflatoxins cause important losses to the poultry industry due to reduced performance and health problems in the exposed birds. The results presented in Table 3, 4 show that AFB1 (500 ng AFB1/g of feed) significantly decreased WG and ADFI, increased FCR and MR, and induced negative changes in some biochemical parameters in broilers. These findings are following the results found by Hernández-Ramírez et al. (18). The authors reported that an experimental diet contaminated with 470 ng AFB1/g feed produced adverse effects on WG, FCR, MR, and serum biochemistry in broiler chickens at 21 days of age. Comparable results are also reported by other researchers (22–26). Moreover, the addition of the YCW product to the AFB1-contaminated diet did not alleviate the harmful effects caused by this mycotoxin. Still, it improved WG and FCR during the final stage of the experiment (14–21 days). These results confirm that the YCW preparation effect was undoubtedly due to its ability to adsorb AFB1, since one of the significant advantages of the glucan-base fractions in animal feeding is to interact with certain mycotoxins. In this context, several in vitro and in vivo reports have indicated that glucan-based binders prevent the toxic effects of different mycotoxins (6–8, 25, 27–32). In the current work, since the minimum manufacturers' recommended inclusion rate was utilized (0.05% w/w), the moderate efficacy of the YCW product to alleviate the adverse effects of AFB1 could be due to its saturation with the mycotoxin. Therefore, diets may need to be supplemented with YCW levels higher than 0.05% to achieve significant protective effects against 500 ng AFB1/g of feed.
The present research revealed positive effects of the YCW product on broiler performance (Table 3). These findings are in close agreement with earlier reports with broilers (33–39). The improved production performance in the YCW group might be related to an improvement in the apparent metabolizable energy intake (40), to the ability of the YCW preparation to stimulate broilers' immunity (41), and to the effects of YCW on disease resistance and gut health (42, 43). The last statement is more plausible because, in this research, the results of the YCW fraction on broiler performance may also be explained by its influence on duodenal histomorphology. In this context, the YCW preparation increased villus height, villus area, crypt depth, and the number of goblet cells in the villi of broiler chickens (Table 5). Similar results have been reported by different researchers (33, 35, 44–46).
Intestinal health is important for broiler performance. When it is impaired, gut histomorphology and gut barrier are damaged. In this sense, different in vivo studies have demonstrated that aflatoxins compromise the gastrointestinal tract's fundamental functions, including loss of barrier function (18, 47, 48). In the present study, intestinal permeability was significantly increased in the AF group (Figure 5), since birds presented a considerable increment in serum FITC-d concentration (up to 0.42 ± 0.05 μg/mL serum). However, the YCW fraction's addition to the AFB1-contaminated diet significantly diminished the serum levels of FITC-d (0.25 ± 0.03 μg/mL serum). These results confirm that the YCW fraction counteracted—to some extent—the AFB1-related toxic damage to the intestinal epithelium in broiler chickens. In this work, the insoluble property and structural conformation allowed β-glucans to adsorb AFB1 molecules mitigating their impact on the gastrointestinal tract. Unfortunately, the YCW fraction did not improve the intestinal epithelium's turnover and regeneration speed in birds of the AF + YCW group (Table 5). However, in addition to a significant increment in the villus height, a higher density of goblet cells was recorded in chickens of the AF + YCW group when compared with the AF group (62.1 vs. 32.9 cell/500 μm). These findings also support the idea that the YCW product alleviates the toxic effects of AFB1 on some histomorphological parameters of the duodenum. Furthermore, compared to the control group, a higher density of goblet cells was also recorded in chickens of the YCW group, suggesting that the YCW fraction can induce the proliferation of goblet cells (Table 5). Different authors have also reported an increased density of goblet cells in broilers-fed diets containing YCW (36, 49). Goblet cells are responsible for the synthesis, storage, and secretion of mucin—a high molecular weight glycoprotein—which represents the first line of defense of the small intestine against mycotoxins (50). In general, the quantity of mucin secreted is directly proportional to the number of goblet cells in villi. Consequently, in this research, an increase in the number of goblet cells in chickens of the AF + YCW group can be positively considered in view of mucus, protective effect against AFB1. Data on the effects of AFB1 on intestinal mucus production in broilers are still meager. However, Wu et al. (51) investigated the individual and combined effect of AFM1 (12 μM), ochratoxin A (20 μM), and zearalenone (100 μM) on the secretion of mucin-like glycoproteins in Caco-2/HT29-MTX cocultures. The authors found that double- and triple-mycotoxin combinations significantly reduced the expression of the highly glycosylated gel-forming mucins MUC2 and MUC5B. As a result, the researchers concluded that increased intestinal permeability is associated with a decrease in mucin secretion. To our knowledge, this is the first report on the effect of AFB1 (500 ng/g feed) on gut histomorphology and gut barrier in broiler chickens with low dietary supplementation of a commercial YCW product (0.5 g/kg). However, further in vivo studies will help improve our understanding of the link between AFB1, mucus production, and intestinal permeability in poultry.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Internal Committee for Care and Use of Experimental Animals of the Postgraduate Program in Animal Production and Health Sciences of the National Autonomous University of Mexico. Ethical approval code: CICUAE-C20_5.
Author Contributions
JH-R, AV-D, and AM-A conceived and designed the experiment and wrote the paper. JH-R acquired, analyzed, and interpreted the data, and performed the statistical analysis. RM-G and GT-I took part in the discussion and helped in editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by Consejo Nacional de Ciencia y Tecnologia (CONACyT) and Programa Interno de Apoyo para Proyectos de Investigacion (PIAPI) grant numbers 299804 and PIAPI-2001.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
JH-R acknowledges CONACyT for the Ph.D. scholarship (245747).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.677965/full#supplementary-material
References
1. Luo Y, Liu X, Li J. Updating techniques on controlling mycotoxins-a review. Food Control. (2018) 89:123–32. doi: 10.1016/j.foodcont.2018.01.016
2. Vila-Donat P, Marín S, Sanchis V, Ramos A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem Toxicol. (2018) 114:246–59. doi: 10.1016/j.fct.2018.02.044
3. Abd El-Hack ME, Samak DH, Noreldin AE, El-Naggar K, Abdo M. Probiotics and plant-derived compounds as eco-friendly agents to inhibit microbial toxins in poultry feed: a comprehensive review. Environ Sci Pollut Res. (2018) 25:31971–86. doi: 10.1007/s11356-018-3197-2
4. Nava-Ramírez MJ, Salazar AM, Sordo M, López-Coello C, Téllez-Isaías G, Méndez-Albores A, et al. Ability of low contents of biosorbents to bind the food carcinogen aflatoxin B1 in vitro. Food Chem. (2021) 345:128863. doi: 10.1016/j.foodchem.2020.128863
5. Joannis-Cassan C, Tozlovanu M, Hadjeba-Medjdoub K, Ballet N, Pfohl-Leszkowicz A. Binding of zearalenone, aflatoxin B1, and ochratoxin A by yeast-based products: a method for quantification of adsorption performance. J Food Prot. (2011) 74:1175–85. doi: 10.4315/0362-028X.JFP-11-023
6. Mendieta CR, Gómez GV, Del Río JCG, Cuevas AC, Arce JM, Ávila EG. Effect of the addition of saccharomyces cerevisiae yeast cell walls to diets with mycotoxins on the performance and immune responses of broilers. Poult Sci J. (2018) 55:38–46. doi: 10.2141/jpsa.0170019
7. Oliveira AA, Keller KM, Deveza MV, Keller LAM, Dias EO, Martini-Santos BJ, et al. Effect of three different anti-mycotoxin additives on broiler chickens exposed to aflatoxin B1. Arch Med Vet. (2015) 47:175–83. doi: 10.4067/S0301-732X2015000200008
8. Liu N, Wang J, Jia S, Chen Y, Wang J. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult Sci. (2018) 97:477–84. doi: 10.3382/ps/pex342
9. Yiannikouris A, André G, Poughon L, François J, Dussap C-G, Jeminet G, et al. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules. (2006) 7:1147–55. doi: 10.1021/bm050968t
10. Jouany J-P, Yiannikouris A, Bertin G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch Zootech. (2005) 8:26–50.
11. Walter PP, Tünde P, István P. Mycotoxins-prevention and decontamination by yeasts. J Basic Microbiol. (2015) 55:805–18. doi: 10.1002/jobm.201400833
12. Ramales-Valderrama RA, Vázquez-Durán A, Méndez-Albores A. Biosorption of B-aflatoxins using biomasses obtained from formosa firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins. (2016) 8:218. doi: 10.3390/toxins8070218
13. Zavala-Franco A, Hernández-Patlán D, Solís-Cruz B, López-Arellano R, Tellez-Isaias G, Vázquez-Durán A, et al. Assessing the aflatoxin B1 adsorption capacity between biosorbents using an in vitro multicompartmental model simulating the dynamic conditions in the gastrointestinal tract of poultry. Toxins. (2018) 10:484. doi: 10.3390/toxins10110484
14. Méndez-Albores A, Arambula-Villa G, Loarca-Piña M, Castano-Tostado E, Moreno-Martínez E. Safety and efficacy evaluation of aqueous citric acid to degrade B-aflatoxins in maize. Food Chem Toxicol. (2005) 43:233–8. doi: 10.1016/j.fct.2004.09.009
15. NRC. Nutrient Requirements of Poultry. 9th rev. ed. Washington, DC: National Academy Press (1994).
16. Jardon-Xicotencatl S, Díaz-Torres R, Marroquín-Cardona A, Villarreal-Barajas T, Méndez-Albores A. Detoxification of aflatoxin-contaminated maize by neutral electrolyzed oxidizing water. Toxins. (2015) 7:4294–314. doi: 10.3390/toxins7104294
17. Baxter MF, Merino-Guzman R, Latorre JD, Mahaffey BD, Yang Y, Teague KD, et al. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front Vet Sci. (2017) 4:56. doi: 10.3389/fvets.2017.00056
18. Hernández-Ramírez J, Nava-Ramírez M, Merino-Guzmán R, Téllez-Isaías G, Vázquez-Durán A, Méndez-Albores A. The effect of moderate-dose aflatoxin B 1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. (2020) 36:31–9. doi: 10.1007/s12550-019-00367-7
20. Lavaisse LM, Hollmann A, Nazareno MA, Disalvo EA. Zeta potential changes of Saccharomyces cerevisiae during fermentative and respiratory cycles. Colloids Surf B: Biointerfaces. (2019) 174:63–9. doi: 10.1016/j.colsurfb.2018.11.001
21. Bowen WR, Sabuni HA, Ventham TJ. Studies of the cell-wall properties of Saccharomyces cerevisiae during fermentation. Biotechnol Bioeng. (1992) 40:1309–18. doi: 10.1002/bit.260401104
22. Miazzo R, Peralta M, Magnoli C, Salvano M, Ferrero S, Chiacchiera S, et al. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult Sci. (2005) 84:1–8. doi: 10.1093/ps/84.1.1
23. Miazzo R, Rosa C, Carvalho EDQ, Magnoli C, Chiacchiera S, Palacio G, et al. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult Sci. (2000) 79:1–6. doi: 10.1093/ps/79.1.1
24. Rauber R, Dilkin P, Giacomini L, de Almeida CA, Mallmann C. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult Sci. (2007) 86:1620–4. doi: 10.1093/ps/86.8.1620
25. Zhao J, Shirley R, Dibner J, Uraizee F, Officer M, Kitchell M, et al. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult Sci. (2010) 89:2147–56. doi: 10.3382/ps.2009-00608
26. Khadem A, Sharifi S, Barati M, Borji M. Evaluation of the effectiveness of yeast, zeolite and active charcoal as aflatoxin absorbents in broiler diets. Global Vet. (2012) 4:426–32.
27. Santin E, Paulillo A, Krabbe E, Alessi A, Polveiro W, Maiorka A. Low level of aflatoxin in broiler at experimental conditions. Use of cell wall yeast as adsorbent of aflatoxin. Arch Vet Sci. (2003) 8:51–5. doi: 10.5380/avs.v8i2.4035
28. Santin E, Paulillo A, Nakagui L, Alessi A, Maiorka A. Evaluation of yeast cell wall on the performance of broiles fed diets with or without mycotoxins. Rev Bras Cienc Avic. (2006) 8:221–5. doi: 10.1590/S1516-635X2006000400004
29. Yiannikouris A, François J, Poughon L, Dussap C-G, Bertin G, Jeminet G, et al. Alkali extraction of β-D-glucans from Saccharomyces cerevisiae cell wall and study of their adsorptive properties toward zearalenone. J Agric Food Chem. (2004) 52:3666–73. doi: 10.1021/jf035127x
30. Stanley VG, Ojo R, Woldesenbet S, Hutchinson DH, Kubena LF. The use of Saccharomyces cerevisiae to suppress the effects of aflatoxicosis in broiler chicks. Poult Sci. (1993) 72:1867–72. doi: 10.3382/ps.0721867
31. Hamza Z, El-Hashash M, Aly S, Hathout A, Soto E, Sabry B, et al. Preparation and characterization of yeast cell wall beta-glucan encapsulated humic acid nanoparticles as an enhanced aflatoxin B1 binder. Carbohydr Polym. (2019) 203:185–92. doi: 10.1016/j.carbpol.2018.08.047
32. Zhao L, Feng Y, Wei J-T, Zhu M-X, Zhang L, Zhang J-C, et al. Mitigation effects of bentonite and yeast cell wall binders on AFB1, DON, and OTA induced changes in laying hen performance, egg quality, and health. Toxins. (2021) 13:156. doi: 10.3390/toxins13020156
33. Muthusamy N, Haldar S, Ghosh T, Bedford M. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Br Poult Sci. (2011) 52:694–703. doi: 10.1080/00071668.2011.633072
34. Gao J, Zhang H, Yu S, Wu S, Yoon I, Quigley J, et al. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult Sci. (2008) 87:1377–84. doi: 10.3382/ps.2007-00418
35. Pascual A, Pauletto M, Giantin M, Radaelli G, Ballarin C, Birolo M, et al. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens. J Anim Sci Biotechnol. (2020) 11:1–11. doi: 10.1186/s40104-020-00448-z
36. Reisinger N, Ganner A, Masching S, Schatzmayr G, Applegate TJ. Efficacy of a yeast derivative on broiler performance, intestinal morphology and blood profile. Livest Sci. (2012) 143:195–200. doi: 10.1016/j.livsci.2011.09.013
37. Santin E, Maiorka A, Macari M, Grecco M, Sanchez J, Okada T, et al. Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. J Appl Poultry Res. (2001) 10:236–44. doi: 10.1093/japr/10.3.236
38. Ghosh T, Haldar S, Bedford M, Muthusami N, Samanta I. Assessment of yeast cell wall as replacements for antibiotic growth promoters in broiler diets: effects on performance, intestinal histo-morphology and humoral immune responses. J Anim Physiol Anim Nutr. (2012) 96:275–84. doi: 10.1111/j.1439-0396.2011.01155.x
39. Fowler J, Hashim M, Haq A, Bailey C. Yeast cell wall and live yeast products and their combination in broiler diets formulated with weekly ingredient variations. J Anim Physiol Anim Nutr. (2015) 99:932–7. doi: 10.1111/jpn.12330
40. Yang Y, Iji P, Kocher A, Thomson E, Mikkelsen L, Choct M. Effects of mannanoligosaccharide in broiler chicken diets on growth performance, energy utilisation, nutrient digestibility and intestinal microflora. Br Poult Sci. (2008) 49:186–94. doi: 10.1080/00071660801998613
41. Brown G, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. (2001) 413:36–7. doi: 10.1038/35092620
42. Li X, Chen Y, Cheng Y, Yang W, Wen C, Zhou Y. Effect of yeast cell wall powder with different particle sizes on the growth performance, serum metabolites, immunity and oxidative status of broilers. Anim Feed Sci Technol. (2016) 212:81–9. doi: 10.1016/j.anifeedsci.2015.12.011
43. Shanmugasundaram R, Sifri M, Selvaraj RK. Effect of yeast cell product supplementation on broiler cecal microflora species and immune responses during an experimental coccidial infection. Poult Sci. (2013) 92:1195–201. doi: 10.3382/ps.2012-02991
44. Baurhoo B, Goldflus F, Zhao X. Purified cell wall of Saccharomyces cerevisiae increases protection against intestinal pathogens in broiler chickens. Int J Poult Sci. (2009) 8:133–7. doi: 10.3923/ijps.2009.133.137
45. Baurhoo B, Phillip L, Ruiz-Feria C. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult Sci. (2007) 86:1070–8. doi: 10.1093/ps/86.6.1070
46. Kim SW, Holanda DM, Gao X, Park I, Yiannikouris A. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: impact on gut health, microbiome, and growth. Toxins. (2019) 11:633. doi: 10.3390/toxins11110633
47. Chen X, Naehrer K, Applegate T. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult Sci. (2016) 95:1312–25. doi: 10.3382/ps/pew022
48. Tejada-Castañeda Z, Avila-Gonzalez E, Casaubon-Huguenin M, Cervantes-Olivares R, Vásquez-Peláez C, Hernández-Baumgarten E, et al. Biodetoxification of aflatoxin-contaminated chick feed. Poult Sci. (2008) 87:1569–76. doi: 10.3382/ps.2007-00304
49. Alizadeh M, Rodriguez-Lecompte J, Rogiewicz A, Patterson R, Slominski B. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance, gut morphology, and gene expression of pattern recognition receptors and cytokines in broiler chickens. Poult Sci. (2016) 95:507–17. doi: 10.3382/ps/pev362
50. Gill N, Wlodarska M, Finlay BB. Roadblocks in the gut: barriers to enteric infection. Cell Microbiol. (2011) 13:660–9. doi: 10.1111/j.1462-5822.2011.01578.x
51. Wu C, Gao Y, Li S, Huang X, Bao X, Wang J, et al. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A. and zearalenone individually or collectively. Toxicol Lett. (2019) 309:1–9. doi: 10.1016/j.toxlet.2019.03.010
Keywords: broilers, AFB1, yeast cell wall, intestinal permeability, histomorphology
Citation: Hernández-Ramírez JO, Merino-Guzmán R, Téllez-Isaías G, Vázquez-Durán A and Méndez-Albores A (2021) Mitigation of AFB1-Related Toxic Damage to the Intestinal Epithelium in Broiler Chickens Consumed a Yeast Cell Wall Fraction. Front. Vet. Sci. 8:677965. doi: 10.3389/fvets.2021.677965
Received: 08 March 2021; Accepted: 21 June 2021;
Published: 26 July 2021.
Edited by:
Shourong Shi, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Yosra Ahmed Soltan, Alexandria University, EgyptF. Capela e Silva, University of Evora, Portugal
Guanhong Li, Jiangxi Agricultural University, China
Copyright © 2021 Hernández-Ramírez, Merino-Guzmán, Téllez-Isaías, Vázquez-Durán and Méndez-Albores. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abraham Méndez-Albores, albores@unam.mx
 Juan Omar Hernández-Ramírez
Juan Omar Hernández-Ramírez Rubén Merino-Guzmán
Rubén Merino-Guzmán Guillermo Téllez-Isaías
Guillermo Téllez-Isaías Alma Vázquez-Durán
Alma Vázquez-Durán Abraham Méndez-Albores
Abraham Méndez-Albores