Abstract
Background: Characterization of reticulo-endothelial activation in COVID-19 may guide treatment. Objectives: To assess reticulo-endothelial activation and its correlation with disease severity and death in patients across the entire spectrum of COVID-19 severity. Methods: Consecutive hospitalized COVID-19 patients were studied, with similar number of patients in each disease severity category. Baseline serum ferritin, sCD163 (macrophage activation markers) and plasma von Willebrand factor (VWF) antigen (endothelial activation marker) levels were studied. Clinical parameters and plasma D-dimer levels were also studied. The study parameters were correlated with COVID-19 severity and survival. Results: The 143 patients (104 males [80%], age 54 [42 – 65] years, median [inter-quartile range]) presented 4 (3—7) days after symptom onset. Thirty-four patients had mild disease, 36 had moderate disease, 36 had severe disease and 37 had critical disease at baseline. With increasing COVID-19 severity, ferritin, sCD163, VWF and D-dimer levels significantly increased at baseline, however, 139 patients had normal sCD163 levels. Of the reticulo-endothelial markers, VWF level independently correlated with COVID-19 severity and with survival. VWF level > 332.6 units/dl correlated with COVID-19 severity (odds ratio [OR]: 2.77 [95% confidence interval (C.I): 1.1 – 6.99], p value: 0.031) and in-hospital death (OR [95% CI]: 29.28 [5.2 – 165], p value < 0.001). Conclusions: Reticulo-endothelial activation markers increased incrementally with worsening COVID-19 severity. Baseline endothelial activation marker (VWF), and not macrophage activation markers, independently correlated with COVID-19 severity and death.
Similar content being viewed by others
Introduction
The innate immune response is the immediate response of the human host to the invading SARS CoV2 virus. Over-active innate immune response may partly explain COVID-19 pathogenesis. Reticulo-endothelial system is one of the arms of the innate immune response. The reticulo-endothelial cells are constituted by macrophages residing in the tissue (on electron microscopy, tissue macrophages appear net-like or reticulated, hence they are termed “reticulo” cells), monocytes and neutrophils in the blood stream and endothelial cells lining blood vessel wall.
On meta-analysis, levels of serum ferritin (marker of macrophage activation) were significantly higher in non – survivors compared to survivors in hospitalized COVID-19 patients [1, 2]. Baseline levels of plasma von Willebrand factor (VWF) (marker of endothelial activation) were raised in hospitalized COVID-19 patients [3,4,5,6], were higher in patients who died compared to survivors [4], and predicted death [3]. A recent review, documents raised VWF along-with low ADAMTS13 (VWF cleaving enzyme—a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) in COVID-19 patients [7]. CD163 (scavenger receptor for hemoglobin haptoglobin complex), a macrophage specific protein, is also a macrophage activation marker shown to be elevated in inflammatory conditions [8, 9].
Varga et al. [10], showed direct endothelial infection with endotheliitis, leading to endothelial dysfunction in COVID-19 patients. In this study, we further explore reticulo-endothelial activation in entire spectrum of COVID -19.
This study aimed to assess degree of reticulo-endothelial activation and its possible contribution to disease severity and to death in patients across the entire spectrum of severity of COVID-19. We hypothesized that plasma VWF levels, serum ferritin and soluble CD163 (sCD163) levels are elevated and may predict death in moderate, severe and critical COVID-19 patients. In this study, we assayed markers of activation of macrophages (serum ferritin, serum sCD163) and of endothelium (plasma VWF antigen level) in COVID-19 patients with the following specific objectives:
-
a) To assess the degree of baseline reticulo-endothelial activation.
-
b) To correlate reticulo-endothelial activation with COVID-19 severity at baseline.
-
c) To correlate baseline reticulo-endothelial activation with in – hospital survival.
Methods
Consecutive adult patients (aged > 18 years) hospitalized with nasopharyngeal swab RT-PCR confirmed SARS CoV-2 infection were recruited after a written informed consent. As per the local governmental regulations at the time of study, all symptomatic individuals with COVID-19, including mild, were admitted. Relevant clinical information pertaining to disease severity and co-morbidities was recorded. COVID-19 severity was categorized as per WHO clinical ordinal score into mild (symptomatic, no pneumonia or hypoxia), moderate (pneumonia, oxygen saturation (SpO2) ≥ 90% on room air), severe (pneumonia with respiratory rate > 30 per min or respiratory distress or SpO2 < 90% on room air) and critical (adult respiratory distress syndrome, sepsis or septic shock) [11].
Laboratory Evaluation for Reticulo-Endothelial Activation
Besides routine laboratory investigations for COVID-19 patients including D—dimer, baseline blood samples were processed for plasma VWF antigen, serum sCD163, and ferritin. The blood samples were collected prior to initiation of any specific therapy for COVID-19 (like steroids, anticoagulation or antivirals). The blood sample for plasma VWF antigen assay was collected using 0.109–M citrate anticoagulant and centrifuged at 2500 g for 15 min at 4 °C. VWF antigen (in-house ELISA-based method, coefficient of variation: 10.7%, lower limit of detection: 6.2 unit/dl, normal range: 50–150 units/dl) and sCD163 [Human CD163 Duo Set ELISA, DY1607, normal range: < 0.98 μg/ml, based on mean + 2SD (0.48 + 0.5) of 28 blood bank control samples] assays were performed by ELISA. The laboratory staff were blinded to the clinical status and disease severity of the patient.
Management and Follow up
All patients were triaged as per WHO score for severity of COVID-19 [9], and managed as per institutional protocol. The in-hospital outcome of these patients was noted.
Sample size
We expected the correlation (Spearman Rank) between the reticulo-endothelial markers and the disease severity categories in COVID-19 patients to be about 0.8. Assuming that this would be significantly better than 0.5 with alpha and beta errors at 5% and 20% respectively, we estimated we needed to study a minimum of 30 patients in each disease category (i.e. sample size of 120, with study subjects in 4 categories of disease severity).
Statistical methods
On admission to hospital, clinical parameters (age, gender, presence of co-morbidities) and biological parameters (reticulo-endothelial activation markers [serum ferritin, serum sCD163 and plasma VWF levels] and plasma D-dimer level) were studied and correlated with COVID-19 severity on hospital admission and with in-hospital survival.
Data was entered in google sheets and converted to Excel 2017. Stata version 15 was used to analyze data. Baseline characteristics were summarized as counts and percentage for categorical variables and median and inter-quartile range for continuous variables. Chi square test was used to test the difference in the proportion of categorical variables according to the groups. Mann Whitney U test (2-independent groups) and Kruskal–Wallis (> 2 independent groups) was used to test the differences in median for continuous variables according to groups. Spearman rank correlation was used to assess the degree of correlation between study parameters and severity of COVID-19.
Receiver operating curve (ROC) was used to assess the utility of VWF, ferritin and D-dimer in predicting outcome and also to derive an optimal cutoff for VWF. From prior studies of COVID-19, we took cutoffs for serum ferritin as > 500 ng/ml [12] and for D-dimer as > 500 ng/ml [13].
Univariate analysis and multivariable penalized logistic regression were used to test the significance of study parameters in predicting severity and outcome. To assess the independent contribution of reticulo-endothelial activation markers to the eventual outcome, we adjusted for lung disease severity (SpO2/ fraction of inspired oxygen [SF ratio]) during multivariate analysis. The impact of each factor was reported using odds ratio, 95% confidence interval and p value.
This study was approved by the Institutional Review Board and Ethics Committee.
Results
Demographics of Study Population
Of 143 patients (104 males [80%], age 54 [42 – 65] years, median [inter-quartile range], presented 4 [3–7] days after onset of symptoms) enrolled in the study from 22 July 2020 to 3 August 2020, 34 patients had mild disease, 36 patients had moderate disease, 36 patients had severe disease and 37 patients had critical disease on Day 1 of hospital stay.
Patients were treated with steroids (Inj. Dexamethsone), anti-coagulation (Inj. Enoxaparin) and anti-virals (Inj. Remdesivir) tailored for severity of COVID-19, as per our institutional management protocol. The patients with mild disease did not receive any specific treatment. Ninety-five patients received steroids (moderate: 24, severe: 36, critical: 35), 95 patients received anticoagulation (moderate: 24, severe: 36, critical: 35) and 23 patients received Remdesivir (moderate: 3, severe: 5, critical: 15).
Patients with moderate disease were treated with steroids: 24 (67%), anticoagulation: 24 (67%) and Remdesivir: 3 (8%). Patients with severe disease were treated with steroids: 36 (100%), anticoagulation: 36 (100%) and Remdesivir: 5 (14%). Patients with critical disease were treated with steroids: 35 (98%), anticoagulation: 35 (98%) and Remdesivir: 15 (42%).
Fifty-two patients (36%) did not require any oxygen support during their admission, 83patients (58%) received various levels of oxygen support, including non-invasive ventilation and 8 patients (6%) had invasive ventilation. Therapeutic limitations, mainly for respiratory support, were faced by 29 patients (20%) (5, 14 and 10 patients each with moderate, severe and critical COVID-19, no patient with mild COVID-19).
Of the 143 patients, 23 patients (16.1%) died during the hospital stay [deaths in patients with mild, moderate, severe and critical disease on Day 1 of hospital stay were 0/34 patients, 3/36 patients (8.3%), 9/36 patients (25%) and 11/37 patients (29.7%) respectively]. Of 29 patients who had therapeutic limitation, 16 patients (55.2%) died.
Degree of Reticulo-Endothelial Activation on Day 1 of Hospital Stay
With increasing COVID-19 severity, statistically significant increase in the levels of serum ferritin, sCD163, plasma VWF antigen and D-dimer was seen on Day 1 of hospital stay (Table 1).
Of the 143 study patients, plasma VWF antigen levels were within normal range in 14 patients (10%), serum ferritin was < 500 ng/ml in 74 patients (52%) and D-dimer was < 500 ng/ml in 60 patients (42%). As serum sCD163 levels were within normal range in 139/143 patients, we excluded it from all further analysis.
Of the 34 patients with mild COVID-19, plasma VWF levels were in normal range in 13 patients (38%), serum ferritin was < 500 ng/ml in 28 patients (82%) and D-dimer was < 500 ng/ml in 32 patients (94%) and sCD163 levels were in normal range in 34 patients (100%).
Significantly higher age, male gender, presence of any co-morbidity, diabetes mellitus or hypertension were also seen with increasing COVID-19 severity (Table 1).
Correlation between Study Parameters on Day 1 of Hospital Stay
The various clinical and biological study parameters correlated with each other and with COVID-19 severity on Day 1 of hospital stay. Plasma VWF, D-dimer and serum ferritin levels showed moderate to strong correlation with each other and with COVID-19 severity. VWF and D-dimer levels showed moderate to strong correlation with age and co-morbidity (data not shown).
Study Parameters as Predictor of Outcome
On ROC analysis, VWF (AUROC: 0.86, 95% CI: 0.79—0.91) was a useful marker predicting mortality with an optimal cutoff of > 332.6 units/dl (sensitivity: 96%, specificity: 78%). Plasma D-dimer (AUROC: 0.76, 95% CI: 0.68—0.83; cutoff > 500 ng/ml—sensitivity: 91% and specificity: 49%) and serum ferritin (AUROC: 0.59, 95% CI: 0.47—0.72; cutoff > 500 ng/ml- sensitivity: 52.4% and specificity: 56%) had lower prognostic performance.
Correlation of study parameters with COVID-19 Severity on Day 1 of Hospital Stay
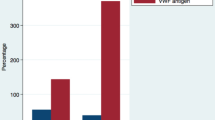
On unadjusted logistic regression, male gender, higher age, presence of any co-morbidity and raised levels of plasma D-dimer, VWF and serum ferritin correlated with COVID-19 severity on Day 1. Figure 1 demonstrates the significantly higher plasma VWF levels in patients with increasing severity of COVID-19.
On adjusted logistic regression, raised levels of plasma D-dimer and VWF correlated with COVID-19 severity on Day 1 (Table 2).
Correlation of Baseline Study Parameters with Risk of in-Hospital Death
In 143 COVID patients, on unadjusted logistic regression, higher age and raised levels of plasma VWF and D-dimer on Day 1 of hospital stay predicted death, (Table 3 and Fig. 2).
On adjusted logistic regression (adjusted for SF ratio), the only significant predictor of in-hospital death was baseline VWF level of > 332.6 units/dl (OR [95% CI]: 29.28 [5.2–165], p value < 0.001) (Table 3).
After excluding 29 patients who faced therapeutic limitations, in remaining 114 COVID-19 patients, on un-adjusted logistic regression, only significant predictor of death was VWF level > 332.6 units/dl on Day 1 of hospital stay (OR [95% CI]: 56.33 [3.09 – 1024], p value: 0.006). Multiple logistic regression could not be performed due to inadequate events.
Discussion
Our study design- with similar number of patients in mild, moderate, severe and critical COVID-19—provides a snapshot of the entire spectrum of COVID-19 severity, in patients who presented 4 (3—7) days after onset of symptoms. Baseline plasma VWF antigen, serum ferritin, sCD163 and D-dimer levels were elevated incrementally with worsening COVID-19 severity; however, serum sCD163 levels were within normal range in 97% of patients. Plasma VWF and D-dimer levels correlated independently with COVID-19 severity at baseline, while only baseline plasma VWF level independently predicted in–hospital survival.
How do we explain the lack of significant macrophage activation in our study? It is possible that macrophage activation syndrome occurs only in a subset of adult patients with severe/critical COVID-19 [14]. Another explanation is that hemophagocytic lympho-histiocytosis, a component of macrophage activation syndrome, is a localized phenomenon in the lungs, without systemic manifestations in adult COVID-19 patients [14, 15].
D-dimer elevation, a typical feature of COVID-19 associated coagulopathy, indicates thrombin generation [16]. Clinical and biological parameters in our study were closely inter-linked.
The main causes of acute lung injury in COVID-19 are thought to be alveolar damage and pulmonary microvascular thrombi. Our study shows endothelial factor (plasma VWF level) to be a significant independent predictor of death in COVID-19 patients. Apart from VWF, other endothelial activation markers like soluble thrombomodulin and angiopoietin-2 are reported to have prognostic significance in COVID-19 [3, 17].
Studies excluding COVID-19 patients facing therapeutic limitation help understand pathogenic mechanisms in those who die, despite receiving maximal respiratory support [18]. On analyzing 114 patients who received maximal therapy in our study, VWF level remained significant predictor of survival; however, as only seven of these patients died, this analysis needs to be viewed with caution. Our study suggests that raised plasma VWF levels predict survival when data from all COVID-19 study patients was analyzed and also, when the analysis was restricted to patients who did not face therapeutic limitation.
It is not clear from our study if the raised plasma VWF levels are due to endothelial injury (as the virus attaches to or invades endothelial cells) or endothelial activation (as part of innate immune response) or both. It is not known if treatment with anti-virals or steroids will reduce the raised VWF levels in COVID-19 patients. Normal levels of macrophage activation markers in patients with mild COVID-19 (serum ferritin < 500 ng/ml in 82% of patients, sCD163 levels < 0.98 μg/ml in 100% of patients) in our study supports the policy of not using steroids to treat mild COVID-19 [19].
Use of plasma VWF antigen as a clinical test to guide management in COVID-19 patients’ needs consideration. VWF is the largest sized molecule in normal human plasma [20]. It is possible that macromolecules and cell debris from activated/injured endothelium in COVID-19 patients may clog the filter formed by the pulmonary microcirculation and lead to hypoxemia [21]. Further mechanistic studies are needed to evaluate the exact role of VWF in pathogenesis and severity of COVID-19 infection.
Although our study suggests that plasma VWF levels are associated with poor prognosis, we do not know whether strategies to reduce plasma VWF levels will alter the natural course of disease. Further studies on evaluating such treatment modalities—N-acetyl cysteine [22, 23] (cleaves disulphide bonds linking VWF dimers), fresh frozen plasma/ cryo-supernatant infusion (provides VWF cleaving enzyme called a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 [ADAMTS13]) [24] and therapeutic plasma exchange (VWF-pheresis as well as provides ADAMTS13 supplementation) [25, 26], can be considered. Recent studies have explored the role of therapeutic plasma exchange in tackling thrombo-inflammation in COVID-19 patients [27].
Our study has a few limitations. We studied reticulo-endothelial markers only at baseline, serial assays of these markers may help better understand disease pathogenesis and treatment response. We measured only VWF antigen levels in our study. Other VWF assays like activity assays (collagen binding activity, ristocetin cofactor activity) and sizes of VWF multimers need to be studied in COVID-19. Although in our study, VWF level predicted outcome even after excluding patients who had therapeutic limitations (n = 114), this sub-analysis needs to be viewed with caution due to limited events (death = 7).
In conclusion, in this study of COVID-19 patients across the spectrum of disease severity, reticulo-endothelial activation markers increased incrementally with worsening COVID-19 severity. Baseline endothelial activation marker (plasma VWF) independently correlated with COVID-19 severity and death; in contrast, macrophage activation markers did not. Better insight into reticulo-endothelial activation at different phases of COVID-19 illness may guide treatment and improve outcomes. In particular, further studies into role of endotheliopathy in COVID-19 progression and therapies to counter it are needed.
References
Khinda J, Janjua NZ, Cheng S, van den Heuvel ER, Bhatti P, Darvishian M. (2020) Association between markers of immune response at hospital admission and COVID-19 disease severity and mortality: A meta-analysis and meta-regression. J Med Virol. 10:https://doi.org/10.1002/jmv.26411
Taneri PE, Gómez-Ochoa SA, Llanaj E, et al (2020) Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 35(8):763–773. https://doi.org/10.1007/s10654-020-00678-5
Goshua G, Pine AB, Meizlish ML, et al (2020) Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol.;7(8):e575-e582. https://doi.org/10.1016/S2352-3026(20)30216-7
Ladikou EE, Sivaloganathan H, Milne KM, et al. (2020) Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond). Sep;20(5):e178-e182. https://doi.org/10.7861/clinmed.2020-0346.
Rauch A, Labreuche J, Lassalle F, et al. (2020) Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.15067.
Helms J, Tacquard C, Severac F, et al (2020) CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med.;46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x.
Favaloro EJ, Henry BM, Lippi G. (2021) Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis. Semin Thromb Hemost. 47(4):400–418. https://doi.org/10.1055/s-0041-1727282.
Buechler C, Eisinger K, Krautbauer S. (2013) Diagnostic and prognostic potential of the macrophage specific receptor CD163 in inflammatory diseases. Inflamm Allergy Drug Targets.;12(6):391–402. https://doi.org/10.2174/18715281113126660060
Vijayalekshmi B, Sharma A, Prabhu SB, et al. (2021) Reticuloendothelial activation correlates with disease severity and predicts mortality in severe alcoholic hepatitis. Eur J Gastroenterol Hepatol. https://doi.org/10.1097/MEG.0000000000002056.
Varga Z, Flammer AJ, Steiger P, et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2;395(10234):1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5.
World Health Organization. (2020). Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332196. License: CC BY-NC-SA 3.0 IGO
Lin Z, Long F, Yang Y, Chen X, Xu L, Yang M. (2020) Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 81(4):647–679. https://doi.org/10.1016/j.jinf.2020.06.053
Guan WJ, Ni ZY, Hu Y, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med.;382(18):1708‐1720. https://doi.org/10.1056/NEJMoa2002032
Prilutskiy A, Kritselis M, Shevtsov A, et al. (2020) SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol. Sep 8;154(4):466–474. https://doi.org/10.1093/ajcp/aqaa124.
Clark KEN, Nevin WD, Mahungu T, Lachmann H, Singh A. (2020) Assessment of the Haemophagocytic lymphohistiocytosis HScore in patients with COVID-19. Clin Infect Dis. Sep 28:ciaa1463. https://doi.org/10.1093/cid/ciaa1463.
Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. (2020) Hematological findings and complications of COVID-19. Am J Hematol. 95(7):834–847. https://doi.org/10.1002/ajh.25829
Smadja DM, Guerin CL, Chocron R, et al. (2020) Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 23(4):611–620.https://doi.org/10.1007/s10456-020-09730-0.
Vincent JL, Taccone FS. (2020) Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 8(5):430–432. https://doi.org/10.1016/S2213-2600(20)30165-X
COVID-19 treatment guidelines. Bethesda, MD (2020) National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/ . Accessed on 15th August 2020
Stockschlaeder M, Schneppenheim R, Budde U.(2014) Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 25(3):206–16. https://doi.org/10.1097/MBC.0000000000000065.
Zachariah U, Nair SC, Goel A, et al. (2020) Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: Consider low volume plasma exchange and low dose steroid. Thromb Res. 192:2.https://doi.org/10.1016/j.thromres.2020.05.001.
Goel A, Nair SC, Zachariah U, et al. (2020) Targetting the raised von Willebrand factor levels in liver diseases: opening up newer therapeutic avenues. EMJ Hepatol. :https://doi.org/10.33590/hepatol/20-0005
Chen J, Reheman A, Gushiken FC, et al. (2011) N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 121(2):593–603. https://doi.org/10.1172/JCI41062
Elias JE, Mackie I, Eapen CE, Chu P, Shaw JC, Elias E. (2013) Porto-pulmonary hypertension exacerbated by platelet transfusion in a patient with ADAMTS13 deficiency. J Hepatol. 58(4):827–30.https://doi.org/10.1016/j.jhep.2012.11.003.
Zheng XL. (2015) ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med.66:211–25. https://doi.org/10.1146/annurev-med-061813-013241.
Zhang L, Zhai H, Ma S, Chen J, Gao Y. (2020) Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br J Haematol. 26 : https://doi.org/10.1111/bjh.16890.
Arulkumaran N, Thomas M, Brealey D, et al. (2020) Plasma exchange for COVID-19 thrombo-inflammatory disease. EJHaem. 30:https://doi.org/10.1002/jha2.140.
Acknowledgements
The authors wish to acknowledge the guidance provided by Dr. Elwyn Elias (Professor Emeritus, Liver Unit, Queen Elizabeth Hospitals, Birmingham, UK) and Dr. KA Balasubramanian (retd. Professor, Wellcome Trust Research labs, Division of GI Sciences, Christian Medical College, Vellore, India) into exploring the link between microangiopathy and organ failure.
Funding
Funding support from FLUID research funds, Christian Medical College, Vellore, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
Concept and design: VV Thomas, SE Kumar, V Alexander, B Vijayalekshmi, S Prabhu, S Kumar, K Murugabharathy, SM Thomas, S Hansdak, R Carey, R Iyyadurai, K Pichamuthu, KPP Abhilash, GM Varghese, S Nair, A Goel, L Jeyaseelan, U Zachariah, A Zachariah, CE Eapen—Analysis: VV Thomas, SE Kumar, V Alexander, A Nadaraj, A Goel, L Jeyaseelan, U Zachariah, A Zachariah, CE Eapen—Interpretation of data: VV Thomas, SE Kumar, V Alexander, A Nadaraj, B Vijayalekshmi, S Prabhu, S Nair, A Goel, L Jeyaseelan, U Zachariah, A Zachariah, CE Eapen—Critical writing/ revising the intellectual content: VV Thomas, SE Kumar, V Alexander, B Vijayalekshmi, S Prabhu, K Murugabharathy, SM Thomas, S Hansdak, R Carey , KPP Abhilash, GM Varghese, S Nair, U Zachariah, A Zachariah, CE Eapen—Final approval: VV Thomas, SE Kumar, V Alexander, A Nadaraj, B Vijayalekshmi, S Prabhu, S Kumar, K Murugabharathy, SM Thomas, S Hansdak, R Carey, R Iyyadurai, K Pichamuthu, KPP Abhilash, GM Varghese, S Nair, A Goel, L Jeyaseelan, U Zachariah, A Zachariah, CE Eapen.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no relevant conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, V.V., Kumar, S.E., Alexander, V. et al. Plasma Von Willebrand Factor Levels Predict Survival in COVID-19 Patients Across the Entire Spectrum of Disease Severity. Indian J Hematol Blood Transfus 38, 333–340 (2022). https://doi.org/10.1007/s12288-021-01459-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01459-0