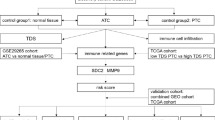
Abstract
Lymph node metastases are strongly associated with unfavorable prognosis in papillary thyroid carcinoma (PTC) patients. However, there are few sensitive or specific indicators that can diagnose or predict lymph node metastases in PTC. The objective of our study was to identify reliable indicators for the diagnosis and prediction of lymph node metastases of PTC. The PTC data set was obtained from The Cancer Genome Atlas (TCGA) cohort. Information on tumor-infiltrating immune cells in PTC was acquired using single-sample gene set enrichment analysis (ssGSEA). Then, the progression-free survival (PFS) rates of PTC patients were evaluated by Kaplan–Meier curves. A tissue microarray including 58 normal thyroid tissues and 57 PTC tissues was processed for CD19 immunohistochemistry staining. Finally, evaluation of phenotype permutations was performed using gene set enrichment analysis (GSEA). There was an appreciable association between immune infiltration and lymph node metastases in PTC. Among those immune cells, B cells and cytotoxic cells showed significant predictive accuracy for lymph node metastases in PTC. Tumor-infiltrating B cells and NK cells were associated with favorable prognosis, while tumor-associated NK CD56bright cells were correlated with poor prognosis in PTC patients. IHC analyses of PTC further confirmed a notably negative correlation between B cell infiltration and lymph node metastases in PTC. Additionally, mutations in BRAF, a dominant cause of tumor mutation burden (TMB), were positively correlated with reduced B cell infiltration and lymph node metastases in PTC. GSEA revealed that epithelial-mesenchymal transition, IL-6/JAK/STAT3 signaling, the inflammatory response, and TNF-α signaling via the NFκB pathway were remarkably suppressed pathways in patients with BRAF mutations. Tumor-associated lymphocytic infiltration, especially B cell infiltration, provides diagnostic and prognostic value for lymph node metastases in PTC.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the article and its supplementary files.
Abbreviations
- AUC:
-
Area under the ROC curve
- GSEA:
-
Gene set enrichment analysis
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- NK:
-
Natural killer
- ROC:
-
Receiver operating characteristic
- ssGSEA:
-
Single-sample gene set enrichment analysis
- TCGA:
-
The Cancer Genome Atlas
- PTC:
-
Papillary thyroid carcinoma
- TMB:
-
Tumor mutation burden
- 95% CI:
-
95% Confidence interval
References
Varricchi G et al (2019) The immune landscape of thyroid cancer in the context of immune checkpoint inhibition. Int J Mol Sci 20(16):3934
Li N et al (2013) Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980–2008. Thyroid 23(1):103–110
Park JL et al (2020) Comprehensive DNA methylation profiling identifies novel diagnostic biomarkers for thyroid cancer.Thyroid 30(2):192–203
Cabrera RN et al (2016) The role of SPECT/CT lymphoscintigraphy and radioguided sentinel lymph node biopsy in managing papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg 142(9):834–841
Liu X et al (2019) Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. J Transl Med 17(1):192
Holland BC et al (2019) Age and sex have no impact on expression levels of markers of immune cell infiltration and immune checkpoint pathways in patients with muscle-invasive urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Immunol Immunother 68(6):991–997
Zhou J et al (2018) Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J Cancer 9(24):4635–4641
Lee HE et al (2011) High FOXP3+ regulatory T-cell density in the sentinel lymph node is associated with downstream non-sentinel lymph-node metastasis in gastric cancer. Br J Cancer 105(3):413–419
Liao H et al (2018) Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett 16(3):3465–3472
Song J et al (2019) Patterns of immune infiltration in HNC and their clinical implications: a gene expression-based study. Front Oncol 9:1285
Lee-Chang C et al (2019) Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res 7(12):1928–1943
Pinto R et al (2019) KRAS-driven lung adenocarcinoma and B cell infiltration: novel insights for immunotherapy. Cancers (Basel) 11(8):1145
Bindea G et al (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39(4):782–795
Chalmers ZR et al (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9(1):34
Xing M et al (2015) Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33(1):42–50
Kim TH et al (2012) The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118(7):1764–1773
Ma S, Song W, Xu Y, Si X, Zhang D, Lv S, Yang C, Ma L, Tang Z, Chen X (2020) Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials 232:119676. https://doi.org/10.1016/j.biomaterials.2019.119676
Yuan Y et al (2016) Role of the tumor microenvironment in tumor progression and the clinical applications (review). Oncol Rep 35(5):2499–2515
Anfray C et al (2019) Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells 9(1):46
Ferrari SM et al (2019) Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci 20(18):4413
Liotti F, Visciano C, Melillo RM (2012) Inflammation in thyroid oncogenesis. Am J Cancer Res 2(3):286–297
Antonelli A, Ferrari SM, Fallahi P (2018) Current and future immunotherapies for thyroid cancer. Expert Rev Anticancer Ther 18(2):149–159
Muppa P et al (2019) Immune cell infiltration may be a key determinant of long-term survival in small cell lung cancer. J Thorac Oncol 14(7):1286–1295
Pages F et al (2018) International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391(10135):2128–2139
Yin H et al (2020) Immune microenvironment of thyroid cancer. J Cancer 11(16):4884–4896
Na KJ, Choi H (2018) Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr Relat Cancer 25(5):523–531
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570
Qian G et al (2020) Thyroid cancer metastasis is associated with an overabundance of defective follicular helper T cells. APMIS 128(8):487–496
Harrell MI, Iritani BM, Ruddell A (2007) Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 170(2):774–786
Miligy I et al (2017) Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 71(2):258–268
Schmidt M et al (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68(13):5405–5413
West NR et al (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13(6):R126
Rody A et al (2009) T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res 11(2):R15
Eiro N et al (2012) Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS One 7(12):e52796
Gu Y et al (2019) Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med 25(2):312–322
Zhang Z et al (2017) Yin-yang effect of tumor infiltrating B cells in breast cancer: from mechanism to immunotherapy. Cancer Lett 393:1–7
Wang SS et al (2019) Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 16(1):6–18
Lee-Chang C et al (2013) Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol 191(8):4141–4151
Aghajani MJ et al (2018) Predictive relevance of programmed cell death protein 1 and tumor-infiltrating lymphocyte expression in papillary thyroid cancer. Surgery 163(1):130–136
Li Y et al (2015) Transforming growth factor beta1 could influence thyroid nodule elasticity and also improve cervical lymph node metastasis in papillary thyroid carcinoma. Ultrasound Med Biol 41(11):2866–2872
Wang N et al (2014) Expression of TGF-beta1, SNAI1 and MMP-9 is associated with lymph node metastasis in papillary thyroid carcinoma. J Mol Histol 45(4):391–399
Howell GM et al (2013) BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol 20(1):47–52
Ylli D et al (2019) Microfluidic droplet digital PCR is a powerful tool for detection of BRAF and TERT mutations in papillary thyroid carcinomas. Cancers (Basel) 11(12):1916
Angell TE et al (2014) BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 24(9):1385–1393
Huang M, Xin W (2018) Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-kappaB/MMPs pathway. Life Sci 192:55–61
Yoo SK et al (2019) Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun 10(1):2764
Wang J et al (2019) Natural naphthohydroquinone dimer rubioncolin C exerts anti-tumor activity by inducing apoptotic and autophagic cell death and inhibiting the NF-kappaB and Akt/mTOR/P70S6K pathway in human cancer cells. Cells 8(12):1593
Acknowledgements
Special thanks to Shanghai Outdo Biotech Co., Ltd., for helping us create the tissue microarray. Thank you to Dr. Ya Xiao for guidance in data analysis.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81602730) and Medical Technology Innovation Fund of Chongqing General Hospital (Y2020ZDXM06).
Author information
Authors and Affiliations
Contributions
Designed the research: ZY, FZ, and WY; acquisition of data: ZY; analysis and interpretation of data: ZY and WY; collection of human PTC and normal thyroid tissue microarray: LY, YZ, YL, HC, and SY; draft of the manuscript: WY and ZY; critical revision of the manuscript: FZ and WY; funding acquisition: LY and ZY. All authors read, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval for this study was granted by the Institute Research Medical Ethics Committee of the Shanghai Outdo Biotech Company. Written informed consent was obtained from all patients.
Consent for publication
Informed consent for publication was obtained from all authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional File 1
Supplementary Figure 1. Coexpression patterns among diverse immune cells. (PDF 48 kb)
Additional File 2
Supplementary Figure 2. Coexpression patterns between B cell infiltration and immunosuppressive markers. (PDF 8 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Yin, L., Zeng, Y. et al. Diagnostic and prognostic value of tumor-infiltrating B cells in lymph node metastases of papillary thyroid carcinoma. Virchows Arch 479, 947–959 (2021). https://doi.org/10.1007/s00428-021-03137-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03137-y