Abstract
Assessing individuals’ abundance, residency (presence at a site within a certain period) and site fidelity (tendency to return to the same site in subsequent seasons or years) is crucial for evaluating and improving the effectiveness of spatial conservation/management measures regarding ecologically and socio-economically valuable species. Using underwater visual census (UVC) and photo-identification (photo-ID) techniques, we estimated the abundance, residency and site fidelity of the dusky grouper, Epinephelus marginatus, at two protected sites within the Tavolara-Punta Coda Cavallo Marine Protected Area (Sardinia, Italy) in the summers of 2017–2018. The scope and spatio-temporal resolution of the study was extended by involving volunteer recreational divers in the photo collection. Grouper mean densities varied significantly across sampling dates, with a significant variability between the 2 years and the two investigated sites. At least 94 grouper visited the study sites in the summers of 2017–2018 based on the analysis of 968 high-quality photos using a semi-automated software to photo-identify individuals. Overall, the most frequently sighted grouper was recorded on 32 different days and 21 individuals (22%) identified in 2017 were re-sighted in 2018. The participation of volunteer recreational divers helped detect the inter-site (3.5–4 km apart) movements of a female and a male, supporting previous findings regarding the occurrence of reproduction-related movements. This study provides novel insights into the residency and site-fidelity patterns of the dusky grouper, and its small-scale movements probably related to reproduction. Specifically, we provide indications that effective protection from fishing should encompass the entire area used by grouper for reproductive movements.
Similar content being viewed by others
Introduction
Marine fish surveys are essential to improve our understanding of the life history traits of species, their spatio-temporal distribution, behaviour, movement and connectivity patterns, and the related causal processes (Pradel 1996; Hammerschlag et al. 2011; Block et al. 2011; Shillinger et al. 2012; Calò et al. 2013; Patterson et al. 2018). Such information is vital for adopting proper management and conservation measures aimed at tackling or mitigating anthropogenic impacts and climate-driven changes threatening fish populations, entire assemblages and the marine ecosystems of which they are a component (Pauly et al. 2005; Worm et al. 2006; Cheung et al. 2009).
Especially in the context of marine protected areas (MPAs), data on fish abundance, residency, site-fidelity and movement range are pivotal to inform conservation practitioners, set/refine protection measures and ultimately ensure the persistence of fish populations (Crowder and Norse 2008; Di Lorenzo et al. 2014, 2016; Di Franco et al. 2018; Germanov et al. 2019; Hays et al. 2019). Many marine fishes can undergo ontogenetic shifts in habitat use (Gillanders et al. 2003) to fulfil important bio-ecological needs that may change through life, such as feeding, sheltering, and spawning (Di Lorenzo et al. 2014, 2016). Many species also aggregate to spawn at predictable times and places, with some of them undertaking seasonal spawning migrations (Sadovy de Mitcheson and Colin 2012; Erisman et al. 2017). Multiple MPA features have been identified as crucial to achieve protection for fish, including the age, the level of protection, compliance and enforcement of the MPA (Claudet et al. 2008; Guidetti et al. 2008; Edgar et al. 2014; Giakoumi et al. 2017). Nevertheless, there is also strong evidence that the effectiveness of MPAs in protecting ecologically and commercially valuable fish species, especially with low mobility, depends on the MPA size relative to the home ranges of species being protected (Kramer and Chapman 1999; Di Franco et al. 2018; Rojo et al. 2019). The bio-ecological effectiveness of MPAs tends to increase when the fishes' reproductive grounds and the spatial scale of species’ movements/migrations, that make them more vulnerable to fishing, are encompassed (Grüss et al. 2011; Sadovy de Mitcheson 2016; Erisman et al. 2017). However, these data are generally lacking for many fish species.
Multiple and diverse assessment methods have been applied to study marine fishes, depending on specific research questions, subjects or hierarchical levels (e.g., assemblage, population, individual). Assemblage- and/or population-based approaches allow investigation of e.g. fish species composition and richness, total and relative abundance, size-frequency distributions, sex-ratios, depth distribution, habitat use and movement patterns. Such data can be obtained either through extractive methods (i.e., catching fishes via different methods), or via non-extractive methods, such as visual sampling (i.e., underwater visual census (UVC) surveys performed by divers, diver-operated or remotely deployed underwater videos, and hydroacoustic techniques; see review by Murphy and Jenkins 2010). All the aforementioned approaches allow for high replication through time and space but some of the main limitations are related to: (1) selectivity bias of fishing gear (extractive methods); (2) observer bias and physiological constraints on dive time and depth in UVC surveys; (3) limited visual field and time-consuming analyses after data collection in video sampling; (4) and the need to ground-truth sonar maps by in situ surveys when using hydroacoustic techniques (Murphy and Jenkins 2010). As a general rule, non-extractive methods and low-impact approaches are more suitable, especially when conducting fish assessments in MPAs (Murphy and Jenkins 2010).
Individual-based approaches allow identification of single individuals, estimation of their size and sex, and tracking them across time and space. In particular, they offer a means to collect information regarding the residency time (duration of individuals’ occupancy at specific sites within a certain period), the degree of fidelity to specific locations (individuals’ tendency to return to the same sites in subsequent seasons/years), and the local movement range of single individuals. Conventional study methods include tagging (via external or internal artificial and chemical marks) and identification through natural marks (such as coloration, pigmentation patterns, spots, morphology, body scars). Tagging is used across multiple marine taxonomic groups and fishes are among the most widely studied taxa (McFarlane et al. 1990; Hazen et al. 2012). Artificial fish tags are invasive to varying degrees, and limitations in their application relate to: (1) material cost and ease of application; (2) mark retention and detectability; (3) potential effects on growth, health and survival of fish (McFarlane et al. 1990). Discriminating individuals based on natural markings require neither their capture nor harm, and is thus far less invasive compared to artificial tags. In particular, given its applicability to threatened and protected species, individual identification via natural markings has received considerable and increasing interest in marine research. This approach exploits any natural and distinctive features which are demonstrated to be persistent over time and to characterize unambiguously specific individuals of a population (Marshall and Pierce 2012). The main limitations of using natural markings are that, in some species, not all the individuals of a population have permanent, clearly distinguishable and unique markings, and difficulties in approaching and/or observing the animals in their natural environment (Marshall and Pierce 2012).
Sketches and/or photographs of natural markings during underwater visual observations can be used as an innocuous means of identification for acquiring information concerning the behaviour of individuals (Porcher 2005; Wall and Herler 2009; Rasotto et al. 2010; Marshall and Pierce 2012). With the development of underwater digital photography, photographic identification (photo ID) has unquestionably become one of the preferred methods for individual recognition in field research (Markowitz et al. 2003). Photo-ID has been applied to the study of several iconic species, first applied to the study of marine mammals, particularly cetaceans, since the 1970s (Markowitz et al. 2003; Hays et al. 2019), then elasmobranchs (Barker and Williamson 2010; Marshall and Pierce 2012; Benjamins et al. 2018; Cerutti-Pereyra et al. 2018; Navarro et al. 2018) and teleost fishes (Wall and Herler 2009; Martin-Smith 2011).
Digital photography is widely practised especially among tourists, who are mostly attracted by charismatic species, some of which are endangered and/or key species whose presence can be considered as an indicator of healthy marine ecosystems and of effective protection measures (Guidetti et al. 2007; Harmelin 2013). The existence of popular destinations for tourism (diving) in MPAs, the greater accessibility of digital photography for all (recreational SCUBA and skin divers, and fishers), and the increasing use of photo-ID as a research tool are broadening the opportunities for the public to become directly involved in scientific projects (Marshall and Pierce 2012; Benjamins et al. 2018; Cerutti-Pereyra et al. 2018; Germanov et al. 2019). Via photo-submission, these collaborative initiatives can enhance the quantity and geographical extent of available data (Holmberg et al. 2008; Barker and Williamson 2010; Benjamins et al. 2018), while simultaneously offering an educational experience to participants.
In the Mediterranean Sea, one of the most iconic and endangered fish species is the dusky grouper, Epinephelus marginatus (Lowe 1834) (Harmelin 2013; Condini et al. 2017; Pollard et al. 2018; Froese and Pauly 2020). Several studies have highlighted that dusky grouper are more abundant and larger in size in fully protected MPAs than in nearby unprotected areas (Di Franco et al. 2009; Sala et al. 2012; Guidetti et al. 2014), potentially enhancing reproductive success and promoting population replenishment. The dusky grouper reproduces in aggregations and spawns in pairs (Zabala et al. 1997b), but spawning aggregations (consisting of tens of individuals) have been rarely observed in the Mediterranean basin, mostly within MPAs (Louisy and Culioli 1999; Pelaprat 1999). This species is known for its strong site-fidelity and small home range (Chauvet and Francour 1990; Lembo et al. 1999; Pastor et al. 2009; Afonso et al. 2011), but small-scale movements/migrations to reproductive sites have also been reported (Zabala et al. 1997a; Hereu et al. 2006). In particular, large dominant males establish mating territories at the beginning of summer and patrol them until the end of the reproductive season, corresponding to late September in the Western Mediterranean Sea (Zabala et al. 1997a, b; Condini et al. 2017).
Limited information is available concerning the extent to which MPAs are effective in protecting key reproductive sites. To date, the only species of Mediterranean bony fish for which a photo-ID technique has been demonstrated to be feasible is E. marginatus (Lelong 1999), but the procedure has never been applied to the study of its populations.
By means of a combination of techniques (i.e., UVC and photo-ID involving both scientists and volunteer recreational divers) providing complementary data, the aims of this study are (1) to estimate the abundance, (2) to assess the residency and site fidelity, and (3) to highlight potential movements of the dusky grouper during its reproductive season between the diving sites located within the MPA of Tavolara-Punta Coda Cavallo (Sardinia Island, Italy; Western Mediterranean).
Materials and methods
Study sites and study species
This study took place in the Tavolara-Punta Coda Cavallo MPA (hereafter TPCC MPA), located in North-Eastern Sardinia (Italy, Western Mediterranean Sea). TPCC MPA was established in 1997 due to its significant natural and landscape values, but regulations were not enforced until 2003–2004. TPCC is a multiple-use MPA; its total surface area (153.57 km2) is divided into three types of sub-zone characterized by different protection regimes, ranging from no-take/no-access zones (A Zones) to buffer areas (B and C Zones), where fishing (professional and recreational) is allowed and regulated. Within A Zones, access is permitted only for research activities, surveillance, and for safety reasons. Diving and local artisanal fishing are allowed within B and C Zones, while regulated recreational fishing is authorized only inside the C Zone. Trawling and spearfishing are prohibited in the entire MPA. The capture of all grouper species by recreational fishers is also forbidden within the C Zone.
The TPCC MPA is considered to be among the MPAs where enforcement and surveillance are effective, which is attested by the significant and persistent “reserve effect” on fishes reported by a number of published studies (Di Franco et al. 2009; Guidetti et al. 2014). Since its establishment, TPCC has promoted the recovery of large high-level predators such as the dusky grouper, E. marginatus. The species shows remarkably high densities in A Zones (Di Franco et al. 2009) and, especially, around protected rocky banks (reported to be spawning grounds, Sahyoun et al. 2013).
Dusky groupers can be individually identified on the basis of the natural, distinctive and permanent patterns of their cephalic blotches (Fig. 1, Lelong 1999). However, as commonly found in grouper, E. marginatus individuals can exhibit ephemeral colour patterns (eight liveries; Zabala et al. 1997b) that change within a split second, affecting the degree of detectability of their permanent marks (Culioli and Quignard 1999). Lateral cephalic patterns are highly recognisable when fish exhibit the standard mottled colour pattern (colour phase ordinarily displayed by the fish, Fig. 1A), but they are also distinguishable in male individuals displaying the silver streaked livery, which is associated with courtship activity (Fig. 1B; Zabala et al. 1997b).
Left side pictures of the same individual male E marginatus (M-SP-002) exhibiting a, c the standard mottled and b the silver streaked colour patterns. c Fingerprint generated by I3S for the picture a. White dots indicate the three reference points, which define the region of interest used for individual identification, while red circles indicate the 33 automatically extracted key points (see text). Pictures were taken in summer 2018 (a, c on 7 August; b on 10 July)
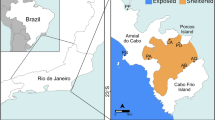
The TPCC MPA comprises an indented coastline of 76 km opposite an archipelago of islands and islets, possessing extensive sandy and sea-grass habitats scattered with rocky outcrops. In this study, UVC and photo-ID were used as complementary non-invasive methods during SCUBA surveys targeting E. marginatus. Field observations and photo-collection were carried out by scientist–divers at two locations, “Molarotto” and “Secche Papa”, located in the A and B Zones of the TPCC MPA, respectively. These two locations were selected as appropriate sites to collect data on the occurrence of the dusky grouper and to locate potential inter-site (< 5 km) movements associated with the reproduction of this species, as they were identified as spawning sites on the basis of the available literature (Sahyoun et al. 2013) and local knowledge (S. Vitale, personal communication). The TPCC MPA seafloor was mapped in 2011–12 with the resolution of 1 m/pixel using a multibeam echosounder by Andromède Oceanologie, within the frame of the PIM (Initiative pour les Petites Iles de Méditerranée—www.initiative-pim.org) project (Fig. 2).

Source QGIS (version 2.18.23), while the slope maps were retrieved using the 3D GIS desktop viewer TerraExplorer Pro (Skyline Software Systems Inc.)
Tavolara–Punta Coda Cavallo MPA zonation map. Main study sites (closed square) and extra dive locations (closed circles 1 and 2), from where two identified individuals moved, are indicated. Three-dimensional slope maps (perspective view looking toward the southwest) of the main study sites are also provided: a slope map of Secche Papa rocky banks; b slope map of Molarotto rocky outcrops (the light blue triangle is an artefact due to the survey vessel mapping the seafloor). The zonation map was created using the Free and Open
The site of Molarotto (A Zone) takes its name from the nearby granitic rocky islet surrounded by countless rocky outcrops protruding from a large sandy area. The site is characterized by granitic boulders and rocky ridges, where the steepest and highest crest-shaped rocky outcrop rises to 20 m from around 30 m depth. The investigated site has a three-dimensional terrain surface area of approximately 3860 m2 (2D area = 3500.46 m2; data retrieved from TerraExplorer Pro, Skyline Software Systems Inc.).
The site of Secche Papa (B Zone) consists of two series of limestone pinnacles, mostly covered with coralligenous formations, rising from the sandy bottom at a depth of about 45 m up to a maximum of 15 m depth. At this site, the approximate three-dimensional terrain surface area that was investigated—within the 35 m isobath—is 3305 m2 (2D area = 2283.41 m2, TerraExplorer Pro, Skyline Software Systems Inc.). The outstanding underwater seascapes and the rich biodiversity characterizing these rocky banks have made this location a renowned Mediterranean diving site, where fishing is prohibited within a radius of 100 m. Taking this into account as well as the popularity of diving tourism in this MPA (Micheli and Niccolini 2013), the features of this location favour the involvement of recreational scuba divers in data collection and educational projects.
Field-work surveys were carried out during two consecutive summers (2017 and 2018) at the two above-mentioned sites. Data collection was spatio-temporally extended owing to the involvement of recreational scuba divers within the frame of a collaborative project (see below in the “photographs collection” section). In particular, volunteer recreational divers enabled the collection of pictures taken at Secche Papa as well as at other diving sites within the B and C zones of the TPCC MPA. Only two of these additional sites were included in this study, since individuals that were identified at Secche Papa were also photographed therein.
Underwater visual census
In the summer period (May–October) of 2017 and 2018, three trained scientist divers collectively performed UVCs to estimate abundances (average density) of dusky grouper at the two study sites. At each site, four replicates of a standard 25 × 5 m2 strip transect were randomly performed on rocky habitat (Harmelin-Vivien et al. 1985). The number and size of the strip transects were determined on the basis of the relatively restricted area to be surveyed and on the limited diving time at the sampled depth range. Within each transect, the actual number and size (using 5-cm size classes) of E. marginatus were recorded.
A total of 84 transects (n = 28 in 2017 and n = 56 in 2018) were carried out at Molarotto (21–30 m depth range), while 116 transects (n = 48 in 2017 and n = 68 in 2018) were conducted at Secche Papa (15–35 m depth range). UVCs were performed during morning hours (0800–1300 h Central European Summer Time; CEST; UTC + 2 h). All times hereafter are referenced to CEST.
Putative differences between the two sampling sites, in time and between the 2 years, in the density of E. marginatus were tested by univariate analyses using PERMANOVA based on Euclidean distance, since the test makes no explicit assumption regarding the distribution of the variable (Anderson 2017). As there were no specific hypotheses about the levels of the factors considered in the study, the factors ‘site’ and ‘year’ were treated as random, while the ‘time of sampling’ (i.e., the different sampling dates, listed here as sequential numbers in progressive order) was treated as a random factor nested in the ‘site’ × ‘year’ interaction, as sampling dates were different at the two study sites except on two occasions. PERMANOVA procedures enable analysis of unbalanced sampling designs such as the one used here. All analyses were performed using PRIMER 6 and PERMANOVA + package (Anderson et al. 2008).
Collection of photographs
In addition to UVC surveys, scientist scuba divers also conducted in situ observations of E. marginatus behaviour at the two main study sites (Molarotto and Secche Papa). Underwater direct observations were performed during 116 dives (n = 35 in 2017; n = 81 in 2018), during three main time slots: after sunrise (n = 32, dives from 0600 to 0900 h), during daytime (n = 22, dives from 0901 to 1300 h), and before sunset (n = 62, dives from 1700 to 2100 h). Individual behaviours, colour patterns and, whenever possible, the corresponding/associated sex were noted on an underwater board, while photos and video footage was taken using a high-definition (HD) camera (Sony Cyber-shot DSC-RX100). Grouper tend to display only one side of their head at a time while maintaining eye contact with the observer. Whenever possible, an attempt to take photos on both sides of individuals was made by swimming around the fish or waiting for it to change profile. Individuals were identified as males when displaying the silver streaked colour pattern associated with courtship behaviour and as females when courted by males (Zabala et al. 1997a). In all other cases with no supporting evidence, sex was recorded as ‘unspecified’. Other features, such as markings and/or damaged fins, were recorded and used as further identification features over short time-periods (e.g. during the same summer). A difference in the frequency of observation between females and males was tested with a two-sample Wilcoxon (or Mann–Whitney) test. We performed the analysis using the wilcox.test() function from the base package of R 4.0.3 (Venables et al. 2020).
Overall, 4398 photos and ≈16 h of video were collected in 2017 and 2018 during field-work surveys at the two study sites. In addition, other underwater pictures recorded with HD resolution at Secche Papa and other diving sites from 2006 to 2018 (n = 70 between 2006 and 2016; n = 128 in 2017; n = 124 in 2018) were added to the digital archive. During summer 2018, recreational scuba divers were also engaged to share past photos or to take pictures at multiple diving sites located within the TPCC MPA. For this study, volunteer divers contributed to the photo collection with 124 pictures and 15 snapshots from videos taken at Secche Papa and at two other diving sites (site 1 and 2, Fig. 2), between which two grouper were observed moving. Furthermore, 48 public pictures found on Facebook were also collected. These photos were shared via e-mail, with clear indication of the site where they had been taken and the date. Poor-quality images shared by recreational divers were discarded (see details below) to maintain data quality and consistency within the dataset. From the resulting digital archive, a total of 968 high-quality photographs/video frames (taken from 2006 to 2018) were selected for the identification of grouper individuals by cephalic blotches and software matching.
Photo-ID: matching software
The software used to store grouper photographs in a digital library and to recognize as well as compare lateral cephalic patterns was the Interactive Individual Identification System I3S Pattern (Version 4.0.2; Den Hartog & Reijns 2014), freely available from the I3S website (http://www.reijns.com/i3s). I3S Pattern has been used in fingerprinting studies of marine and terrestrial reptile species such as sea turtles (Chelonia mydas and Eretmochelys imbricata, Calmanovici et al. 2018) and lizards (Liopholis slateri, Treilibs et al. 2016).
I3S Pattern is a semi-automated matching software that requires user interaction. The user must point out three fixed reference points and identify the relevant region bearing the individually identifiable pattern of the unknown animal. In this study, the edge of the upper lip, the anterior base of the dorsal fin and the opercular caudal edge were manually entered and used as reference points (Fig. 1). Once the reference points are entered, the software automatically extracts all key points located within the reference area, generating a unique fingerprint for each image. To match fingerprints, the software compares between the key point patterns of any new unknown individual (test image) and known individuals (catalogued images), ranking every image from the database based on how closely the sizes and locations of key point pairs match. In particular, the numerical ranking, termed the distance metric, is calculated from the sum of the distances between each key point pair, divided by the square of the number of key point pairs (Den Hartog and Reijns 2014). The lower is the score, the better is the match.
Since automatic key point extraction is sensitive to visual circumstances (i.e., water clarity, uneven natural lighting on the fish, reflections, backscatter—particles illuminated by the flash), poor-quality photographs were a priori excluded to reduce incorrect matches. High-quality images were defined as those that (1) portrayed individuals with the standard mottled or silver streaked colour patterns, (2) contained the reference area, (3) were clear and sharp, and (4) were taken with an approximately perpendicular viewing angle with respect to the animal.
The software interface allows detailed visual comparison of an unknown image against a ranked list of photos from the database, thus allowing the user to check the match, include the image of a confirmed individual in the database, and to prevent the co-identification of different individuals (i.e., false positive error, Stevick et al. 2011).
To test the validation process performed with the software and to reduce the likelihood of failing to match two photographs of the same individual (i.e., false negative error, Stevick et al. 2011), pattern matching performance was investigated using multiple identifying features for the identification (scars and/or damaged fins) and by manual comparison of all the pictures in the database. After having completed the catalogue, a reference image per individually identified grouper was selected and compared by eye against the entire identification database twice. This exhaustive comparison in the image library also allowed the detection of false negative errors, and thus the matching of a few photographs by eye. Data about the accuracy of I3S Pattern in the photo-recognition of the dusky grouper are not provided as this goes beyond the scope of this work.
Results
Underwater visual census
At both sites and during the summers of 2017 and 2018, densities displayed a high temporal variability. Overall, grouper average densities ranged from a minimum of 0.5 ± 0.3 (mean ± SE, n = 4) to a maximum of 7.7 ± 2.5 (n = 4) individuals per 125 m2. At both sites, the average density values were higher in 2018 than in 2017 (Fig. 3).
Average density estimates obtained via UVC (a, c, e, g) and number of grouper identified through photo-ID (b, d, f, h) at each of the two study sites (Molarotto and Secche Papa) in 2017 and 2018. UVC data (a, c, e, g). Average density values (points) ± standard error (SE, bars) of grouper per UVC transect (n = 4 in all cases except on 30 October 2017 at Secche Papa when n = 8). Photo-ID (b, d, f, h). Number of individuals photo-identified per day
In summer 2017, overall average densities of 1.5 ± 0.5 (n = 28) and 2.3 ± 0.4 (n = 48) individuals per 125 m2 were found at Molarotto and Secche Papa, respectively, while in summer 2018 these values were 5.3 ± 0.4 (n = 56) individuals at Molarotto and 4.1 ± 0.4 (n = 68) individuals per 125 m2 at Secche Papa. Statistical analyses showed a significant effect (P < 0.05) of the factor ‘time’ and of the interaction ‘site’ × ‘year’ (Table 1). In other words, average densities varied significantly across sampling dates and between the 2 years, but the differences were site-specific.
Photo ID
At both study sites, the number of individuals identified from the right side is greater than those identified from the left side. Only for a few individuals were pictures of both sides taken (Table 2). To avoid double counting, because we cannot rule out that pictures taken from different sides on different days may belong to the same individual, only right-side pictures (both sides for the individuals for which both pictures were available) were considered, thus providing a conservative and minimum estimate of the total number of grouper. Based on the 968 pictures analysed (taken in the 2006–2018 period), the minimum number of grouper that have visited or resided at the study sites during the summer seasons of 2017 and 2018 is 94 (Table 2). Considering only the summers of 2017 and 2018, 76 and 18 individuals were photo-identified from the right side at Secche Papa (374 pictures, 0.2 individuals per picture) and Molarotto (49 pictures, 0.3 individuals per picture), respectively (Table 2).
In 2017 and 2018, overall, 43 individuals were photographed only on one calendar day, while the remaining 51 were recorded on several different days, varying between 2 and 32 (Figs. 4, 5).
Out of the 76 individuals identified from the right side at Secche Papa, 12 were identified as males and 7 as females (Fig. 4), while at Molarotto only 2 individuals out of 18 were identified as males (Fig. 5). For the other individuals it was not possible to determine the sex. At Secche Papa, males were sighted significantly more frequently than females (Fig. 6, Wilcoxon or Mann–Whitney test, W = 3898.5, P = 0.015).
The number of identified individuals recorded per day and the frequency of observations over time are greater at Secche Papa than Molarotto. At least 30 individuals were recorded on three different days at Secche Papa in 2017 and 2018 (Fig. 4), compared to the four individuals that were sighted on at least three different days at Molarotto during the same period (Figs. 5, 8). At Secche Papa, 11 individuals, for which past pictures were available, have been sighted before 2017 and two of them were first photographed in 2010.
Overall, 21 individuals out of the 94 (22%) were identified in 2017 and re-sighted in 2018. At Secche Papa, 20 individuals out of the 76 (26%) that have been identified were recorded in 2017 and re-sighted in 2018 (Fig. 7).
Temporal occurrences of the 20 photo-ID individuals recorded during both consecutive summers at Secche Papa. The time frames corresponding to the summers of 2017 and 2018 are enlarged in two subgraphs. The sex (F = female, M = male; U = unspecified) of these individuals is indicated before the alphanumeric ID codes listed on the y-axis. Different colours indicate different individuals, which are listed in ascending order of number of sightings from top to bottom
At Molarotto, only one grouper out of the 18 individuals identified was recorded during both summers 2017 and 2018 (Fig. 8).
Temporal occurrences of the four photo-ID individuals recorded on at least three different calendar days at Molarotto. The time frame corresponding to the summer of 2018 is enlarged in a subgraph. The sex (M = male; U = unspecified) of these individuals is indicated before the alphanumeric ID codes listed on the y-axis. Different colours indicate different individuals, which are listed in ascending order of number of sightings from top to bottom
Two individuals (a male, Fig. 1, and a female, supplementary Fig. 1) were detected moving between the study site of Secche Papa and two other locations, well known and frequented by divers. The female (F-SP-073, around 85 cm in total length) is readily identified by eye due to her unusual colour pattern and the injured left pectoral fin (supplementary Fig. 1). Therefore, it was possible to use even poor-quality pictures/video snapshots (unidentifiable with the software but clearly distinguishable by eye) and divers’ reports. This female was observed and photographed at Secche Papa on three occasions in 2018 but her presence was also reported at another diving site 3.5 linear km away (site 1, Fig. 2) since 8 July 2012. From 2012 to 2018, her presence was recorded during summer on 17 calendar days (7 at Secche Papa, 10 at site 1). This female moved between site 1 and Secche Papa at least once in July 2017 and at least thrice between July and August 2018. The male M-SP-002 (Fig. 1) has been the most recorded grouper, with a total of 32 sightings on different days during the summers of 2017 and 2018 (22 sightings only in 2018, Fig. 7). In 2018, it was first photographed on 16 June 2018, at a diving spot more than 4 linear km away from Secche Papa (site 2, Fig. 2) and then, from 30 June until 23 September 2018, it was only recorded at Secche Papa. Therefore, even if we cannot rule out that this individual moved back and forth from the study site during the summer of 2018, the collected data support strong site-fidelity.
Discussion
This study has provided evidence that dusky grouper displayed (1) significantly variable densities in time and space; (2) strong residency and site-fidelity; and (3) small-scale inter-site movements (3.5–4 km) at the investigated protected sites.
Average densities of grouper estimated by UVC in the present study are in agreement with previous assessments carried out within the TPCC MPA (Guidetti et al. 2007, 2008, 2014; Di Franco et al. 2009; Sala et al. 2012; Sahyoun et al. 2013; Prato et al. 2017) and in other Mediterranean MPAs (Chauvet and Francour 1990; Zabala et al. 1997a, b; La Mesa and Vacchi 1999; Pelaprat 1999; Reñones et al. 1999; Lenfant et al. 2003). At some of these MPAs, dusky grouper spawning aggregations and reproduction were also documented or suggested to occur (Zabala et al. 1997a, b; Pelaprat 1999; Reñones et al. 1999; Marinaro et al. 2005). Therefore, the similarity in such UVC estimates suggests that the protected sites we monitored within the TPCC MPA represent reproductive sites for the dusky grouper. Moreover, the variability in average density recorded over time in the present study might be linked to the movements of individuals out of the study sites and might be small-scale individual reproductive displacements (see below).
Among the different sources of variability in the observed grouper abundance/counts, we must acknowledge the significant variability inherent in strip transect UVC sampling, and instantaneous variation in fish abundance, as documented for both tropical (Kulbicki and Sarramégna 1999; McClanahan et al. 2007) and temperate (Irigoyen et al. 2013) reef fish populations. Sources of variation that may affect the detection of spatio-temporal patterns in fish populations also include fish behavioural response to divers, fish movements linked to currents and the formation of reproductive or feeding aggregations (McClanahan et al. 2007; Irigoyen et al. 2018). Recent studies reported high spatio-temporal variability in the number of medium-to-large sized fish species occurring at low densities and characterised by patchy distributions, such as groupers (Irigoyen et al. 2018; Rojo et al. 2021). Variability in abundance estimates can be reduced, theoretically, by increasing the census area and/or by increasing the number of replicates (Underwood 1997; Kulbicki and Sarramégna 1999; Prato et al. 2017; Irigoyen et al. 2018; Rojo et al. 2021). In our study, we used widely employed 25 × 5 m2 strip transects due to i) the limited spatial extension of the area surveyed at each study site (especially Secche Papa) and ii) the need for an acceptable number of replicated counts to estimate variability (i.e., n = 4 per dive and study site). From this perspective, however, new developments in census methods have recently been proposed to maximize the area covered while reducing count variability, including tracked roaming transects, wider and longer strip transects (35 × 20 or 50 × 5 m2), and the use of distance sampling (Prato et al. 2017; Irigoyen et al. 2018; Rojo et al. 2021). As far as individual abundances are concerned, the present study allowed us to photo-identify a total of 94 individuals of dusky grouper at the study sites over the two consecutive summers (2017 and 2018). A higher number of individuals were identified at Secche Papa than at Molarotto. This difference can be explained by two complementary factors. The first explanation can be attributed to a different photographic collection effort at the two study sites. Both tourist divers and scientist–divers collected pictures at Secche Papa, while only scientists took photos at Molarotto (the latter being a no-take/no-entry zone). In addition, at Secche Papa the sampling effort is likely to have increased during July–August 2017 and 2018, due to a peak in tourist frequentation, leading to a larger contribution by recreational divers. The second explanation relies on grouper being less accustomed to encountering divers at Molarotto than at the diving site of Secche Papa. The ‘wilder’ Molarotto grouper are wary of SCUBA divers, which rapidly escape or hide under boulders, and may negatively influence abundance estimation, especially through photo-ID (difficulty of taking pictures).
Although full sex determination was not possible, and many individuals remained sexually unclassified, the number of photo-identified males recorded at Secche Papa amounted to 7 in 2017 and 11 in 2018. These numbers correspond with those reported at the renowned spawning aggregation of Medes Islands (5–12 males, Hereu et al. 2006; 6 males, Zabala et al. 1997b) and surpassed those found in the spawning aggregation at Punta Revellata, where the presence of only one dominant male was reported (Pelaprat 1999).
In the present research, the contribution of local photographers and tourist divers has been crucial to widen the time window of the study, allowing assessment of the residency and site-fidelity of grouper as well as highlighting individual small-scale movements through photo-ID. Such data have been collected only at Secche Papa, where grouper are more confident around divers and thus easier to photograph.
Regarding grouper site-fidelity and residency, using photo-ID, a total of 21 grouper (22%) identified in 2017 were re-sighted in 2018. In particular, at Secche Papa we recorded the re-sighting of 20 individuals (26%) during the summers of 2017 and 2018, confirming the previously documented strong site-fidelity of the dusky grouper (Chauvet and Francour 1990; Pastor et al. 2009; Afonso et al. 2011; Félix-Hackradt et al. 2013; Hackradt et al. 2014; Koeck et al. 2014). A similar result was obtained using natural markings (morphological anomalies, cephalic blotches, skin wounds, damaged fin rays) to individually identify and re-sight 15 individuals during the summers of 1997 and 1998 in the Natural Reserve of the Strait of Bonifacio (Culioli and Quignard 1999). Because not all individuals identified at Secche Papa in summer 2017 were re-sighted in summer 2018, this may be due to the lack of opportunity to photograph them even where present, the movement of individuals out of the study site, or in a worst case scenario, their capture through fishing. A previous study in Port-Cros National Park highlighted, via a marking study, the partial turnover of small–medium grouper from one summer to the next (Chauvet and Francour 1990).
The most sighted individual was a male, recorded on 32 different days over the summers of 2017 and 2018 at Secche Papa, and also reported therein since 2010. Identified females were recorded on fewer days than males, however, a female individual was first seen in 2010, testifying that females can also exhibit strong site-fidelity to specific locations. Although we cannot state that the female was reproductively active and successfully mated throughout this 8-year period, it is plausible that its recurrent presence at Secche Papa makes this site a reproductive ground. In fact, not only dominant males but also females are thought to visit preferred sites (Zabala et al. 1997a). Although we are unable to determine how long these individuals permanently resided at Secche Papa, this is the longest documented period (8 years) over which individually identified dusky grouper have been recorded on multiple occasions at a given site. These findings correspond with Culioli and Quignard’s (1999) findings from the Lavezzi Islands, where a male was present at the study site, patrolling the same territory, over at least five summers, while five identified females were recorded over two consecutive summers. These results further suggest that cephalic patterns in dusky grouper are stable over periods of at least 8 years, increasing the previous known estimate of 4–5 years (Culioli and Quignard 1999; Lelong 1999). Nonetheless, the stability of these cephalic patterns over the life of individual dusky grouper still needs to be assessed. Although young grouper present clearly visible cephalic blotches that, according to Lelong (1999), should change only in size during growth, it remains to be verified whether photo‐ID can be used for the long‐term monitoring of this species, from the juvenile to the adult stage.
Regarding potential migrations of grouper, we were able to highlight the small-scale movements of two individuals. The male (and the most sighted individual of the study) moved to Secche Papa at the beginning of summer 2018. This further supports the hypothesis proposed by Zabala et al. (1997b) that dusky grouper may undertake a small-scale reproductive migration at the beginning of the reproductive season (late spring) aimed at establishing a summer territory. For the female, we are unable to determine whether her movements back and forth between Secche Papa and the other site were due to a search for shelter, foraging or for reproductive reasons. However, females are known to move between males’ territories (Hereu et al. 2006).
Our observations regarding dusky grouper movements are consistent with previous evidence. The dusky grouper is known to exhibit year-round strong sedentary behaviour (Chauvet and Francour 1990; Koeck et al. 2014) and long residency times within small home ranges with an estimated area of 10,000–30,000 m2 (Lembo et al. 1999; Pastor et al. 2009) or a minimum length of 5.1 km (Afonso et al. 2011). However, dusky grouper are also reported to perform occasional small-scale movements (Chauvet and Francour 1990; Lembo et al. 1999; Pastor et al. 2009; Afonso et al. 2011). Acoustic telemetry studies reported that travel distances of grouper are rarely longer than 1 km from the core-area of their home ranges (Chauvet and Francour 1990; Lembo et al. 1999; Pastor et al. 2009) but a 5-year study in the Azores documented that they could extend up to 3.2 km (Afonso et al. 2011). Therefore, the linear distances travelled by the two grouper in our study (3.5–4 km) fall within the estimated minimum linear range (< 5 km) of the species and, excluding the homing displacement of around 4.4 km documented by Lembo et al. (1999), they are the longest recorded in the literature to date.
Overall, our study highlights the importance of addressing protection benefits across time and space to improve fish conservation and ensure long-term population persistence. Our results on local densities, site-fidelity, residency, and movements of the dusky grouper not only confirm earlier findings but also provide strong and novel indications that one of the investigated sites (Secche Papa) is likely to play a key role for dusky grouper spawning and reproduction. We thus recommend extending the full protection of putative reproductive sites (Secche Papa) to the surroundings areas, over distances of at least a 5 km radius, and on a precautionary basis, to cover the full extent of dusky grouper movements, especially those related to reproduction. Despite the limitations of this study, and although further data are needed to draw more robust conclusions, we believe that our results will help inform managers’ decisions as they are directly applicable to other MPAs, geographic regions and fish species.
Data availability
The database generated and analysed during the current study is available from the corresponding author upon reasonable request and with permission of the rights holders (owners of the photos) and the Marine Protected Area of Tavolara-Punta Coda Cavallo.
References
Afonso P, Fontes J, Santos RS (2011) Small marine reserves can offer long term protection to an endangered fish. Biol Conserv 144:2739–2744. https://doi.org/10.1016/j.biocon.2011.07.028
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Stat Ref Online. https://doi.org/10.1002/9781118445112.stat07841
Anderson M, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods, vol 1. Plymouth, PRMER-E, p 218
Barker SM, Williamson JE (2010) Collaborative photo-identification and monitoring of grey nurse sharks (Carcharias taurus) at key aggregation sites along the eastern coast of Australia. Mar Freshw Res 61:971–979. https://doi.org/10.1071/MF09215
Benjamins S, Dodd J, Thorburn J, Milway VA, Campbell R, Bailey DM (2018) Evaluating the potential of photo-identification as a monitoring tool for flapper skate (Dipturus intermedius). Aquat Conserv Mar Freshw Ecosyst 28:1360–1373. https://doi.org/10.1002/aqc.2937
Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, Hazen EL, Foley DG, Breed GA, Harrison A-L, Ganong JE, Swithenbank A, Castleton M, Dewar H, Mate BR, Shillinger GL, Schaefer KM, Benson SR, Weise MJ, Henry RW, Costa DP (2011) Tracking apex marine predator movements in a dynamic ocean. Nature 475:86
Calmanovici B, Waayers D, Reisser J, Clifton J, Proietti M (2018) I3S Pattern as a mark-recapture tool to identify captured and free-swimming sea turtles: an assessment. Mar Ecol Prog Ser 589:263–268. https://doi.org/10.3354/meps12483
Calò A, Félix-Hackradt FC, Garcia J, Hackradt CW, Rocklin D, Treviño Otón J, Charton JAG (2013) A review of methods to assess connectivity and dispersal between fish populations in the Mediterranean Sea. Adv Oceanogr Limnol 4:150–175. https://doi.org/10.1080/19475721.2013.840680
Cerutti-Pereyra F, Bassos-Hull K, Arvizu-Torres X, WilkinsonI KA, García-Carrillo L, Perez-Jimenez JC, Hueter RE (2018) Observations of spotted eagle rays (Aetobatus narinari) in the Mexican Caribbean using photo-ID. Environ Biol Fishes 101:237–244. https://doi.org/10.1007/s10641-017-0694-y
Chauvet C, Francour P (1990) Le mérous Epinephelus guaza du Parc national de Port-Cros (France): Aspects socio-démographiques. (Social demographic aspects of the grouper Epinephelus guaza of the National Park of Port-Cros (France)). Bull La Soc Zool Fr 114:5–13
Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson R, Pauly D (2009) Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish 10:235–251. https://doi.org/10.1111/j.1467-2979.2008.00315.x
Claudet J, Osenberg CW, Benedetti-Cecchi L, Domenici P, García-Charton J-A, Pérez-Ruzafa Á, Badalamenti F, Bayle-Sempere J, Brito A, Bulleri F, Culioli J-M, Dimech M, Falcón JM, Guala I, Milazzo M, Sánchez-Meca J, Somerfield PJ, Stobart B, Vandeperre F, Valle C, Planes S (2008) Marine reserves: size and age do matter. Ecol Lett 11:481–489. https://doi.org/10.1111/j.1461-0248.2008.01166.x
Condini MV, García-Charton JA, Garcia AM (2017) A review of the biology, ecology, behavior and conservation status of the dusky grouper, Epinephelus marginatus (Lowe 1834). Rev Fish Biol Fish 28:301–330. https://doi.org/10.1007/s11160-017-9502-1
Crowder L, Norse E (2008) Essential ecological insights for marine ecosystem-based management and marine spatial planning. Mar Policy 32:772–778. https://doi.org/10.1016/j.marpol.2008.03.012
Culioli J-M, Quignard J-P (1999) Suivi de la démographie et du comportement territorial des mâles de mérous bruns Epinephelus marginatus (Lowe, 1834) (Pisces, Serranidae) du site du Pellu (Réserve naturelle des Bouches de Bonifacio, Corse, Méditerranée N.O.). 9:3–9
Den Hartog JE, Reijns RA (2014) I3S Pattern manual: interactive individual identification system. Version 4.0.2. 42
Di Franco A, Bussotti S, Navone A, Panzalis P, Guidetti P (2009) Evaluating effects of total and partial restrictions to fishing on Mediterranean rocky-reef fish assemblages. Mar Ecol Prog Ser 387:275–285. https://doi.org/10.3354/meps08051
Di Franco A, Plass-Johnson JG, Di Lorenzo M, Meola B, Claudet J, Gaines SD, García-Charton JA, Giakoumi S, Grorud-Colvert K, Hackradt CW, Micheli F, Guidetti P (2018) Linking home ranges to protected area size: the case study of the Mediterranean Sea. Biol Conserv 221:175–181. https://doi.org/10.1016/j.biocon.2018.03.012
Di Lorenzo M, D’Anna G, Badalamenti F, Giacalone V, Starr R, Guidetti P (2014) Fitting the size of no-take zones to species movement patterns: a case study on a Mediterranean seabream. Mar Ecol Prog Ser 502:245–255. https://doi.org/10.3354/meps10723
Di Lorenzo M, Claudet J, Guidetti P (2016) Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J Nat Conserv 32:62–66. https://doi.org/10.1016/j.jnc.2016.04.004
Edgar GJ, Stuart-Smith RD, Willis TJ, Kininmonth S, Baker SC, Banks S, Barrett NS, Becerro MA, Bernard ATF, Berkhout J, Buxton CD, Campbell SJ, Cooper AT, Davey M, Edgar SC, Försterra G, Galván DE, Irigoyen AJ, Kushner DJ, Moura R, Parnell PE, Shears NT, Soler G, Strain EMA, Thomson RJ (2014) Global conservation outcomes depend on marine protected areas with five key features. Nature 506:216–220. https://doi.org/10.1038/nature13022
Erisman B, Heyman W, Kobara S, Ezer T, Pittman S, Aburto-Oropeza O, Nemeth RS (2017) Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish 18:128–144. https://doi.org/10.1111/faf.12132
Félix-Hackradt FC, Hackradt CW, Treviño-Otón J, Pérez-Ruzafa A, García-Charton JA (2013) Temporal patterns of settlement, recruitment and post-settlement losses in a rocky reef fish assemblage in the South-Western Mediterranean Sea. Mar Biol 160:2337–2352. https://doi.org/10.1007/s00227-013-2228-2
Froese R, Pauly D (2020) FishBase. Epinephelus marginatus (Lowe, 1834). http://www.marinespecies.org/aphia.php?p=taxdetails&id=127036. Accessed 26 Nov 2020
Germanov ES, Bejder L, Chabanne DB, Dharmadi D, Hendrawan IG, Marshall AD, Pierce SJ, van Keulen M, Loneragan NR (2019) Contrasting habitat use and population dynamics of reef manta rays within the Nusa Penida Marine Protected Area, Indonesia. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00215
Giakoumi S, Scianna C, Plass-Johnson J, Micheli F, Grorud-Colvert K, Thiriet P, Claudet J, Di Carlo G, Di Franco A, Gaines SD, García-Charton JA, Lubchenco J, Reimer J, Sala E, Guidetti P (2017) Ecological effects of full and partial protection in the crowded Mediterranean Sea: a regional meta-analysis. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-08850-w
Gillanders BM, Able KW, Brown JA, Eggleston DB, Sheridan PF (2003) Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar Ecol Ser 247:281–295. https://doi.org/10.1016/j.biomaterials.2007.11.012
Grüss A, Kaplan DM, Guénette S, Roberts CM, Botsford LW (2011) Consequences of adult and juvenile movement for marine protected areas. Biol Conserv 144:692–702. https://doi.org/10.1016/j.biocon.2010.12.015
Guidetti P, Bussotti S, Panzalis P, Bava S, Trainito E, Vitale S, Morri C, Cattaneo-Vietti R, Navone A, Bianchi Carlo N (2007) Ecological and management implications of protecting Epinephelus marginatus at the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy; Western Mediterranean Sea). In: Francour P, Gratiot J (eds) 2nd symposium on Mediterranean Groupers, pp 83–86
Guidetti P, Milazzo M, Bussotti S, Molinari A, Murenu M, Pais A, Spanò N, Balzano R, Agardy T, Boero F, Carrada G, Cattaneo-Vietti R, Cau A, Chemello R, Greco S, Manganaro A, Notarbartolo di Sciara G, Russo GF, Tunesi L (2008) Italian marine reserve effectiveness: does enforcement matter? Biol Conserv 141:699–709. https://doi.org/10.1016/j.biocon.2007.12.013
Guidetti P, Baiata P, Ballesteros E, Di Franco A, Hereu B, Macpherson E, Micheli F, Pais A, Panzalis P, Rosenberg AA, Zabala M, Sala E (2014) Large-scale assessment of Mediterranean marine protected areas effects on fish assemblages. PLoS ONE 9:e91841. https://doi.org/10.1371/journal.pone.0091841
Hackradt CW, Garcìa-Charton JA, Harmelin-Vivien M, Pérez-Ruzafa A, Le Diréach L, Bayle-Sempere J, Charbonnel E, Ody D, Renones O, Sanchez-Jerez P, Valle C (2014) Response of rocky reef top predators (Serranidae: Epinephelinae) in and around marine protected areas in the Western Mediterranean Sea. PLoS ONE 9:e98206. https://doi.org/10.1371/journal.pone.0098206
Hammerschlag N, Gallagher AJ, Lazarre DM (2011) A review of shark satellite tagging studies. J Exp Mar Bio Ecol 398:1–8. https://doi.org/10.1016/j.jembe.2010.12.012
Harmelin J-G (2013) Le mérou brun et le corb: deux Grands Témoins de 50 ans de protection du milieu marin dans le Parc national de Port-Cros (France, Méditerranée). Sci Rep Port-Cros Natl Park 277:263–277
Harmelin-Vivien M, Harmelin JG, Chauvet C, Duval C, Galzin R, Lejeune P, Barnabé G, Blane F, Chevalier R, Duclere J, Lasserre G (1985) Evaluation visuelle des peuplements et populations de poissons: méthodes et problémes. (The underwater observation of fish communities and fish populations. Methods and problems). Rev D’écologie La Terre La Vie 40:467–539
Hays GC, Bailey H, Bograd SJ, Bowen WD, Campagna C, Carmichael RH, Casale P, Chiaradia A, Costa DP, Cuevas E, Nico de Bruyn PJ, Dias MP, Duarte CM, Dunn DC, Dutton PH, Esteban N, Friedlaender A, Goetz KT, Godley BJ, Halpin PN, Hamann M, Hammerschlag N, Harcourt R, Harrison AL, Hazen EL, Heupel MR, Hoyt E, Humphries NE, Kot CY, Lea JSE, Marsh H, Maxwell SM, McMahon CR, Notarbartolo di Sciara G, Palacios DM, Phillips RA, Righton D, Schofield G, Seminoff JA, Simpfendorfer CA, Sims DW, Takahashi A, Tetley MJ, Thums M, Trathan PN, Villegas-Amtmann S, Wells RS, Whiting SD, Wildermann NE, Sequeira AMM (2019) Translating marine animal tracking data into conservation policy and management. Trends Ecol Evol 34:459–473. https://doi.org/10.1016/j.tree.2019.01.009
Hazen EL, Maxwell SM, Bailey H, Bograd SJ, Hamann M, Gaspar P, Godley BJ, Shillinger GL (2012) Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar Ecol Prog Ser 457:221–240. https://doi.org/10.3354/meps09857
Hereu B, Diaz D, Pasqual J, Zabala M, Sala E (2006) Temporal patterns of spawning of the dusky grouper Epinephelus marginatus in relation to environmental factors. Mar Ecol Prog Ser 325:187–194. https://doi.org/10.3354/meps325187
Holmberg J, Norman B, Arzoumanian Z, Applications SE, Jan N (2008) Robust, comparable population metrics through collaborative photo-monitoring of whale sharks. Ecol Appl 18:222–233
Irigoyen AJ, Galván DE, Venerus LA, Parma AM (2013) Variability in abundance of temperate reef fishes estimated by visual census. PLoS ONE 8:1–12. https://doi.org/10.1371/journal.pone.0061072
Irigoyen AJ, Rojo I, Calò A, Trobbiani G, Sánchez-Carnero N, García-Charton JA (2018) The “Tracked Roaming Transect” and distance sampling methods increase the efficiency of underwater visual censuses. PLoS ONE 13:1–15. https://doi.org/10.1371/journal.pone.0190990
Koeck B, Pastor J, Saragoni G, Dalias N, Payrot J, Lenfant P (2014) Diel and seasonal movement pattern of the dusky grouper Epinephelus marginatus inside a marine reserve. Mar Environ Res 94:38–47. https://doi.org/10.1016/j.marenvres.2013.12.002
Kramer DL, Chapman MR (1999) Implications of fish home range size and relocation for marine reserve function. Environ Biol Fishes 55:65–79
Kulbicki M, Sarramégna S (1999) Comparison of density estimates derived from strip transect and distance sampling for underwater visual censuses: a case study of Chaetodontidae and Pomacanthidae. Aquat Living Resour 12:315–325. https://doi.org/10.1016/S0990-7440(99)00116-3
La Mesa G, Vacchi M (1999) An analysis of the coastal fish assemblage of the Ustica Island marine reserve (Mediterranean Sea). Mar Ecol 20:147–165. https://doi.org/10.1046/j.1439-0485.1999.00067.x
Lelong P (1999) Identification individuelle du mérou brun, Epinephelus marginatus (Lowe, 1834) par les taches céphaliques. Mar Life 9:29–35
Lembo G, Fleming IA, Økland F, Carbonara P, Spedicato MT (1999) Site fidelity of the dusky grouper Epinephelus marginatus studied by acoustic telemetry. Mar Life 9:37–43
Lenfant P, Louisy P, Licari M (2003) Recensement des mérous bruns (Epinephelus marginatus) de la réserve naturelle de Cerbère-Banyuls (France, Méditerranée) effectué en septembre 2001, après 17 années de protection. (Inventory of dusky groupers (Epinephelus marginatus) in the marine reserve of Cerbère-Banyuls (France, North-Western Mediterranean Sea) after 17 years of protection). Cybium 27:27–36. http://sfi-cybium.fr/fr/recensement-des-m%C3%A9rous-bruns-epinephelus-marginatus-de-la-r%C3%A9serve-naturelle-de-cerb%C3%A8re-banyuls
Louisy P, Culioli J (1999) Synthèse des observations sur l’activité reproductrice du mérou brun Epinephelus marginatus (Lowe, 1834) en Méditerranée nord-occidentale. Mar Life 9:47–57
Marinaro JY, Roussel E, Lawson J, Crech’hriou R, Planes S (2005) Premier signalement d’une reproduction effective du mérou brun, Epinephelus marginatus, dans la Réserve marine de Cerbère-Banyuls (France). (First record of effective reproduction of the dusky grouper, Epinephelus marginatus (Lowe, 1834), in the Cerbere-Banyuls (France) marine protected area). Cybium 29:198–200. http://sfi-cybium.fr/fr/premier-signalement-d%E2%80%99une-reproduction-effective-du-m%C3%A9rou-brun-epinephelus-marginatus-dans-la
Markowitz TM, Harlin AD, Würsig B (2003) Digital photography improves efficiency of individual dolphin identification. Mar Mammal Sci 19:217–223. https://doi.org/10.1111/j.1748-7692.2003.tb01103.x
Marshall AD, Pierce SJ (2012) The use and abuse of photographic identification in sharks and rays. J Fish Biol 80:1361–1379. https://doi.org/10.1111/j.1095-8649.2012.03244.x
Martin-Smith KM (2011) Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. J Fish Biol 78:1757–1768. https://doi.org/10.1111/j.1095-8649.2011.02966.x
McClanahan TR, Graham NAJ, Maina J, Chabanet P, Bruggemann JH, Polunin NVC (2007) Influence of instantaneous variation on estimates of coral reef fish populations and communities. Mar Ecol Prog Ser 340:221–234. https://doi.org/10.3354/meps340221
McFarlane GA, Wydoski RS, Prince ED (1990) Historical review of the development of external tags and marks. Am Fish Soc Symp 7:9–79
Micheli F, Niccolini F (2013) Achieving success under pressure in the conservation of intensely used coastal areas. Ecol Soc 18:19. https://doi.org/10.5751/ES-05799-180419
Murphy HM, Jenkins GP (2010) Observational methods used in marine spatial monitoring of fishes and associated habitats: a review. Mar Freshw Res 61:236–252. https://doi.org/10.1071/MF09068
Navarro J, Perezgrueso A, Barría C, Coll M (2018) Photo-identification as a tool to study small-spotted catshark Scyliorhinus canicula. J Fish Biol 92:1657–1662. https://doi.org/10.1111/jfb.13609
Pastor J, Verdoit-Jarraya M, Astruch P, Dalias N, Nelva Pasqual JS, Saragoni G, Lenfant P (2009) Acoustic telemetry survey of the dusky grouper (Epinephelus marginatus) in the Marine Reserve of Cerbère-Banyuls: informations on the territoriality of this emblematic species. Comptes Rendus Biol 332:732–740. https://doi.org/10.1016/j.crvi.2009.03.010
Patterson TA, Eveson JP, Hartog JR, Evans K, Cooper S, Lansdell M, Hobday AJ, Davies CR (2018) Migration dynamics of juvenile southern bluefin tuna. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-32949-3
Pauly D, Alder J, Bakun A, Heileman S, Kock K-H, Mace P, Perrin W, Stergiou K, Sumaila UR, Vierros M, Freire K, Sadovy Y, Christensen V, Kaschner K, Palomares M-L, Tyedmers P, Wabnitz C, Watson R, Worm B (2005) Marine fisheries systems. Ecosystems and human well-being: current state and trends. Island Press, Washington, D.C., pp 477–511
Pelaprat C (1999) Observations on the spawning behaviour of the dusky grouper Epinephelus marginatus (Lowe, 1834) in the north of Corsica (France). Mar Life 9:59–65
Pollard DA, Afonso P, Bertoncini AA, Fennessy S, Francour P, Barreiros J (2018) Epinephelus marginatus. E. T7859A100467602. In: IUCN red list of threatened species. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T7859A100467602.en
Porcher IF (2005) On the gestation period of the blackfin reef shark, Carcharhinus melanopterus, in waters off Moorea, French Polynesia. Mar Biol 146:1207–1211. https://doi.org/10.1007/s00227-004-1518-0
Pradel R (1996) Utilization of Capure-Mark-Recapture for the study of recruitment and population growth rate. Biometrics 52:703–709
Prato G, Thiriet P, Di Franco A, Francour P (2017) Enhancing fish underwater visual census to move forward assessment of fish assemblages: an application in three Mediterranean marine protected areas. PLoS ONE 12:1–20. https://doi.org/10.1371/journal.pone.0178511
Rasotto MB, De Mitcheson YS, Mitcheson G (2010) Male body size predicts sperm number in the mandarinfish. J Zool 281:161–167. https://doi.org/10.1111/j.1469-7998.2009.00688.x
Reñones O, Goñi R, Pozo M, Deudero S, Moranta J (1999) Effects of protection on the demographic structure and abundance of Epinephelus marginatus (Lowe, 1834). Evidence from Cabrera Archipelago National Park (West-central Mediterranean). Mar Life 9:45–53
Rojo I, Sánchez-Meca J, García-Charton JA (2019) Small-sized and well-enforced marine protected areas provide ecological benefits for piscivorous fish populations worldwide. Mar Environ Res 149:100–110. https://doi.org/10.1016/j.marenvres.2019.06.005
Rojo I, Irigoyen AJ, Cuadros A, Calò A, Pereñíguez JM, Hernández-Andreu R, Félix-Hackradt FC, Carreño F, Hackradt CW, García-Charton JA (2021) Detection of protection benefits for predatory fishes depends on census methodology. Aquat Conserv Mar Freshw Ecosyst. https://doi.org/10.1002/aqc.3539
Sadovy de Mitcheson Y (2016) Mainstreaming fish spawning aggregations into fishery management calls for a precautionary approach. Bioscience 66:295–306. https://doi.org/10.1093/biosci/biw013
Sadovy de Mitcheson Y, Colin PL (2012) Reef fish spawning aggregations: biology, research and management. In: Fish and fisheries series. Springer, New York
Sahyoun R, Bussotti S, Di Franco A, Navone A, Panzalis P, Guidetti P (2013) Protection effects on Mediterranean fish assemblages associated with different rocky habitats. J Mar Biol Assoc United Kingdom 93:425–435. https://doi.org/10.1017/S0025315412000975
Sala E, Ballesteros E, Dendrinos P, Di Franco A, Ferretti F, Foley D, Fraschetti S, Friedlander A, Garrabou J, Güçlüsoy H, Guidetti P, Halpern BS, Hereu B, Karamanlidis A, a, Kizilkaya Z, Macpherson E, Mangialajo L, Mariani S, Micheli F, Pais A, Riser K, Rosenberg A a, Sales M, Selkoe K a, Starr R, Tomas F, Zabala M, (2012) The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7:e32742. https://doi.org/10.1371/journal.pone.0032742
Shillinger GL, Bailey H, Bograd SJ, Hazen EL, Hamann M, Gaspar P, Godley BJ, Wilson RP, Spotila JR (2012) Tagging through the stages: technical and ecological challenges in observing life histories through biologging. Mar Ecol Prog Ser 457:165–170. https://doi.org/10.3354/meps09816
Stevick PT, Palsbøll PJ, Smith TD, Bravington MV, Hammond PS (2011) Errors in identification using natural markings: rates, sources, and effects on capture–recapture estimates of abundance. Can J Fish Aquat Sci 58:1861–1870. https://doi.org/10.1139/f01-131
Treilibs CE, Pavey CR, Hutchinson MN, Bull CM (2016) Photographic identification of individuals of a free-ranging, small terrestrial vertebrate. Ecol Evol 6:800–809. https://doi.org/10.1002/ece3.1883
Underwood AJ (1997) Experiments in ecology: logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Venables W, Smith D, R Core Team (2020) An Introduction to R. Notes on R: a programming environment for data analysis and graphics version 4.0.3 (2020-10-10). Godalming, Network Theory Ltd, p 99
Wall M, Herler J (2009) Postsettlement movement patterns and homing in a coral-associated fish. Behav Ecol 20:87–95. https://doi.org/10.1093/beheco/arn118
Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790. https://doi.org/10.1126/science.1132294
Zabala M, Louisy P, García-Rubies A, Gracia V (1997a) Socio-behavioural context of reproduction in the Mediterranean dusky grouper Epinephelus marginatus (Lowe, 1834) (Pisces, Serranidae) in the Medes Islands Marine Reserve (NW Mediterranean, Spain). Sci Mar 61:79–89
Zabala M, Garcia-Rubies A, Louisy P, Sala E (1997b) Spawning behaviour of the Mediterranean dusky grouper Epinephelus marginatus (Lowe, 1834) (Pisces, Serranidae) in the Medes Islands Marine Reserve (NW Mediterranean, Spain). Sci Mar 61:65–77
Acknowledgements
The authors thank all the divers who contributed photographs and attended public information events. In particular, we would like to thank, in alphabetical order, Eric De Keyser, Luigi Onnis, Gianluca Pecetti, Irina Podda, Luigi Prosperi and Claretta Spada for their significant contributions, without which re-sightings and key information would not otherwise have been possible. We thank Andrea Deiana for his help with GIS. We also thank Jurgen den Hartog and Renate Reijns, the developers of the I3S application, for their helpful advice to use I3S Pattern. Finally, we wish to thank Michael Paul for the revision of the English language.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This work was carried out within the framework of the PhD project of E. Desiderà and the Citizen Science Project “TavolaraLab”, both funded by the Marine Protected Area of Tavolara-Punta Coda Cavallo, Ministero dell’Ambiente e della Tutela del Territorio e del Mare (MATTM). This work was also partially supported by funding from the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) and Consorzio Nazionale Interuniversitario per le Scienze del Mare (CoNISMa).
Author information
Authors and Affiliations
Contributions
ED, CM, and PG conceived the project. AN contributed to the implementation of the research. ED, ET, LM, PP and PG carried out the sampling activity. ED analysed the data and RB aided in data processing and figure design. ED drafted the manuscript and all authors contributed to its review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approvals were required as no animals were handled during this work. All field activities were carried out in compliance with national laws and with the permission to undertake the study within the Marine Protected Area (MPA) issued by Tavolara-Punta Coda Cavallo MPA.
Additional information
Responsible Editor: E. Hunter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by: A. Irigoyen and an undisclosed expert.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desiderà, E., Trainito, E., Navone, A. et al. Using complementary visual approaches to investigate residency, site fidelity and movement patterns of the dusky grouper (Epinephelus marginatus) in a Mediterranean marine protected area. Mar Biol 168, 111 (2021). https://doi.org/10.1007/s00227-021-03917-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03917-9