Abstract
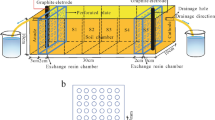
Electrokinetic remediation is a process in which a direct electric current is applied across a section of contaminated soil to remove metals. To improve the electrokinetic remediation in this study, a conductive membrane was fabricated via in situ chemical polymerization employing pyrrole and copper oxide nanoparticles. The fabricated membrane was placed in an electric field as part of the electrode structure. A physical model was constructed and filled with copper-contaminated kaolinite in the concentration of 200 mg/kg. To control the pH, 0.1 M citric acid and 0.01 M potassium chloride were used as the electrolyte solutions. Experimental parameters such as voltage, current, pH, EC, drained flow, and copper concentration were measured. The results showed that the minimum surface resistivity of the fabricated membrane under a maximum pressure of 8.2 kPa was 2.55 kΩ/m2. The experimental results demonstrated that the use of citric acid as an electrolyte was more useful to desorb the copper due to the formation of the copper-citrate complex. When employing the fabricated membrane, the copper removal increased from 13% (in CT-2) to 63% (in GM-2), while the removal of copper using potassium chloride electrolyte increased from 42% (in CT-1) to 52% (in GM-1). The highest power consumption was obtained in experiments using citric acid. Due to the higher removal efficiency of copper in GM-2, the energy utilization efficiency (β) increased and reached 29.9 near β value of GM-1 with the lowest power consumption.














Similar content being viewed by others
Data availability
The data are available upon request from the corresponding author.
Notes
American Association of Textile Chemists and Colorists.
References
Aboukila, E. F., & Norton, J. B. (2017). Estimation of saturated soil paste salinity from soil-water extracts. Soil Science, 182(3), 107–113.
Acar, Y. B., Gale, R. J., Alshawabkeh, A. N., Marks, R. E., Puppala, S., Bricka, M., & Parker, R. (1995). Electrokinetic remediation: Basics and technology status. Journal of Hazardous Materials, 40(2), 117–137.
Almeira, J., Peng, C. S., & Abou-Shady, A. (2012). Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation. Desalination, 300, 1–11.
Apori, O. S., Hanyabui, E., & Asiamah, Y. J. (2018). Remediation technology for copper contaminated soil: A review. Asian Soil Research Journal, 1–7.
Arbabi, M., & Golshani, N. (2016). Removal of copper ions Cu (II) from industrial wastewater: A review of removal methods. International Journal of Epidemiologic Research, 3(3), 283–293.
Ayyanar, A., & Thatikonda, S. (2021). Enhanced electrokinetic removal of heavy metals from a contaminated lake sediment for ecological risk reduction. Soil and Sediment Contamination: An International Journal, 30(1), 12–34.
Bajaj, P., Gupta, A. P., & Ojha, N. (2000). Antistatic and hydrophilic synthetic fibers: A critique. Journal of Macromolecular Science, Part C: Polymer Reviews, 40(2–3), 105–138.
Ballabio, C., Panagos, P., Lugato, E., Huang, J. H., Orgiazzi, A., Jones, A., Fernández-Ugalde, O., Borrelli, P., & Montanarella, L. (2018). Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Science of the Total Environment, 636, 282–298.
Burnotte, F., Lefebvre, G., & Grondin, G. (2004). A case record of electroosmotic consolidation of soft clay with improved soil electrode contact. Canadian Geotechnical Journal, 41(6), 1038–1053.
Cameselle, C., Gouveia, S., & Cabo, A. (2020). Analysis and optimization of mn removal from contaminated solid matrixes by electrokinetic remediation. International Journal of Environmental Research and Public Health, 17(6), 1820.
Cang, L., Zhou, D. M., Alshawabkeh, A. N., & Chen, H. F. (2007). Effects of sodium hypochlorite and high pH buffer solution in electrokinetic soil treatment on soil chromium removal and the functional diversity of soil microbial community. Journal of Hazardous Materials, 142(1–2), 111–117.
Chang, J. H., Shi, Y. H., & Tung, C. H. (2010). Stepwise addition of chemical reagents for enhancing electrokinetic removal of Cu from real site contaminated soils. Journal of Applied Electrochemistry, 40(6), 1153–1160.
Chen, J. L., Yang, S. F., Wu, C. C., & Ton, S. (2011). Effect of ammonia as a complexing agent on electrokinetic remediation of copper-contaminated soil. Separation and Purification Technology, 79(2), 157–163.
De Gioannis, G., Muntoni, A., Polettini, A., & Pomi, R. (2008). Enhanced electrokinetic treatment of different marine sediments contaminated by heavy metals. Journal of Environmental Science and Health Part A, 43(8), 852–865.
EPA, E. (1996). Method 3050B-Acid digestion of sediments, sludges and soils. Test Methods for Evaluating Solid Wastes: Physical/Chemical Methods. EPA SW‐846, 1, 3050B-1.
Eriksson, F. & Gemvik, L. (2014). Electro-Osmotic Treatment of Soil: A laboratory investigation of three Swedish clays.
Ferrero, F., Napoli, L., Tonin, C., & Varesano, A. (2006). Pyrrole chemical polymerization on textiles: Kinetics and operating conditions. Journal of Applied Polymer Science, 102(5), 4121–4126.
Fourie, A. B., & Jones, C. J. F. P. (2010). Improved estimates of power consumption during dewatering of mine tailings using electrokinetic geosynthetics (EKGs). Geotextiles and Geomembranes, 28(2), 181–190.
Fu, R., Wen, D., Xia, X., Zhang, W., & Gu, Y. (2017). Electrokinetic remediation of chromium (Cr)-contaminated soil with citric acid (CA) and polyaspartic acid (PASP) as electrolytes. Chemical Engineering Journal, 316, 601–608.
Giannis, A., & Gidarakos, E. (2005). Washing enhanced electrokinetic remediation for removal cadmium from real contaminated soil. Journal of Hazardous Materials, 123(1–3), 165–175.
Gong, Y., Zhao, D., & Wang, Q. (2018). An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Research, 147, 440–460.
Gray, D. H. (1970). Electrochemical hardening of clay soils. Geotechnique, 20(1), 81–93.
Hakansson, E., Kaynak, A., Lin, T., Nahavandi, S., Jones, T., & Hu, E. (2004). Characterization of conducting polymer coated synthetic fabrics for heat generation. Synthetic Metals, 144(1), 21–28.
Hamzenejad Taghlidabad, R., & Sepehr, E. (2018). Heavy metals immobilization in contaminated soil by grape-pruning-residue biochar. Archives of Agronomy and Soil Science, 64(8), 1041–1052.
Jeyakanthan, V., Gnanendran, C. T., & Lo, S. C. (2011). Laboratory assessment of electro-osmotic stabilization of soft clay. Canadian Geotechnical Journal, 48(12), 1788–1802.
Kabata‐Pendias, A., & Sadurski, W. (2004). Trace elements and compounds in soil. Elements and their compounds in the environment: Occurrence, analysis and biological relevance, 79–99.
Kaynak, A., & Håkansson, E. (2005). Generating heat from conducting polypyrrole-coated PET fabrics. Advances in Polymer Technology: Journal of the Polymer Processing Institute, 24(3), 194–207.
Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 182, 247–268.
Kimura, T., Takase, K. I., & Tanaka, S. (2007). Concentration of copper and a copper–EDTA complex at the pH junction formed in soil by an electrokinetic remediation process. Journal of Hazardous Materials, 143(3), 668–672.
Lamont-Black, J., Jones, C. J. F. P., & Alder, D. (2016). Electrokinetic strengthening of slopes–Case history. Geotextiles and Geomembranes, 44(3), 319–331.
Libohova, Z., Wills, S., Odgers, N. P., Ferguson, R., Nesser, R., Thompson, J. A., West, L. T., & Hempel, J. W. (2014). Converting pH 1: 1 H2O and 1: 2CaCl2 to 1: 5 H2O to contribute to a harmonized global soil database. Geoderma, 213, 544–550.
Liu, L., Li, W., Song, W., & Guo, M. (2018). Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Science of the Total Environment, 633, 206–219.
Lizama-Tzec, F. I., Canché-Canul, L., & Oskam, G. (2011). Electrodeposition of copper into trenches from a citrate plating bath. Electrochimica Acta, 56(25), 9391–9396.
Ma, Q., Li, J., Lee, C. C., Long, X., Liu, Y., & Wu, Q. T. (2019). Combining potassium chloride leaching with vertical electrokinetics to remediate cadmium-contaminated soils. Environmental Geochemistry and Health, 41(5), 2081–2091.
Maity, S. (2017). Optimization of processing parameters of in-situ polymerization of pyrrole on woollen textile to improve its thermal conductivity. Progress in Organic Coatings, 107, 48–53.
Malinauskas, A. (2001). Chemical deposition of conducting polymers. Polymer, 42(9), 3957–3972.
Maturi, K., & Reddy, K. R. (2008). Cosolvent-enhanced desorption and transport of heavy metals and organic contaminants in soils during electrokinetic remediation. Water, Air, and Soil Pollution, 189(1–4), 199–211.
MEF. (2007). Government decree on the assessment of soil contamination and remediation needs 214/2007 (legally binding texts are those in Finnish and Swedish Ministry of the Environment).
Micic, S., Shang, J. Q., Lo, K. Y., Lee, Y. N., & Lee, S. W. (2001). Electrokinetic strengthening of a marine sediment using intermittent current. Canadian Geotechnical Journal, 38(2), 287–302.
Mitchell, J. K., & Soga, K. (2005). Fundamentals of soil behavior (Vol. 3). New York: Wiley.
Niinae, M. & Aoki, K. (2005). Enhanced electrokinetic remediation of cadmium contaminated soils by chelating agents. European Journal of Mineral Processing & Environmental Protection, 5(2).
Nogueira, M. G., Pazos, M., Sanromán, M. A., & Cameselle, C. (2007). Improving on electrokinetic remediation in spiked Mn kaolinite by addition of complexing agents. Electrochimica Acta, 52(10), 3349–3354.
Oorts K. (2013) Copper. In: Alloway B. (Eds.), Heavy metals in soils. environmental pollution, Vol. 22. Springer.
Page, A. L., Miller, R. H., & Keeney, D. R. (1982). Methods of soil analysis. American Society of Agronomy.
Park, S. M., Kim, J. G., Kim, H. B., Kim, Y. H., & Baek, K. (2020). Desorption technologies for remediation of cesium-contaminated soils: a short review. Environmental Geochemistry and Health, 1–10.
Peng, G., & Tian, G. (2010). Using electrode electrolytes to enhance electrokinetic removal of heavy metals from electroplating sludge. Chemical Engineering Journal, 165(2), 388–394.
Pirsa, S. (2013). Fabrication of 1, 1-dimethylhydrazine gas sensor based on nano structure conducting polyaniline. Journal of Sciences, Islamic Republic of Iran, 24(3), 209–215.
Qin, G., Niu, Z., Yu, J., Li, Z., Ma, J. Y., & Xiang, P. (2020). Soil heavy metal pollution and food safety in China: effects, sources and removing technology. Chemosphere, 129205.
Reddy, K. R., & Chinthamreddy, S. (2003). Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soils. Journal of Geotechnical and Geoenvironmental Engineering, 129(3), 263–277.
Reddy, K. R., & Chinthamreddy, S. (2004). Enhanced electrokinetic remediation of heavy metals in glacial till soils using different electrolyte solutions. Journal of Environmental Engineering, 130(4), 442–455.
Rittirong, A., Douglas, R. S., Shang, J. Q., & Lee, E. C. (2008). Electrokinetic improvement of soft clay using electrical vertical drains. Geosynthetics International, 15(5), 369–381.
Rode, S., Henninot, C., Vallières, C., & Matlosz, M. (2004). Complexation chemistry in copper plating from citrate baths. Journal of the Electrochemical Society, 151(6), C405.
Rozas, F., & Castellote, M. (2012). Electrokinetic remediation of dredged sediments polluted with heavy metals with different enhancing electrolytes. Electrochimica Acta, 86, 102–109.
Sadki, S., Schottland, P., Brodie, N., & Sabouraud, G. (2000). The mechanisms of pyrrole electropolymerization. Chemical Society Reviews, 29(5), 283–293.
Sata, T., Yamaguchi, T., & Matsusaki, K. (1996). Preparation and properties of composite membranes composed of anion-exchange membranes and polypyrrole. The Journal of Physical Chemistry, 100(41), 16633–16640.
Skotheim, T. A., & Reynolds, J. (2007). Handbook of conducting polymers, 2. CRC Press.
Song, Y., Cang, L., Zuo, Y., Yang, J., Zhou, D., Duan, T., & Wang, R. (2020). EDTA-enhanced electrokinetic remediation of aged electroplating contaminated soil assisted by combining dual cation-exchange membranes and circulation methods. Chemosphere, 243, 125439.
Torabi, M. S., Asadollahfardi, G., Rezaee, M., & Panah, N. B. (2021). Electrokinetic removal of Cd and Cu from mine tailing: EDTA enhancement and voltage intensity effects. Journal of Hazardous, Toxic, and Radioactive Waste, 25(2), 05020007.
Tsang, D. C., Yip, T. C., & Lo, I. M. (2009). Kinetic interactions of EDDS with soils 2 Metal–EDDS complexes in uncontaminated and metal-contaminated soils. Environmental Science & Technology, 43(3), 837–842.
Virkutyte, J., Sillanpää, M., & Latostenmaa, P. (2002). Electrokinetic soil remediation—Critical overview. Science of the Total Environment, 289(1–3), 97–121.
Wu, J., Zhang, J., & Xiao, C. (2016). Focus on factors affecting pH, flow of Cr and transformation between Cr (VI) and Cr (III) in the soil with different electrolytes. Electrochimica Acta, 211, 652–662.
Yang, J. S., Kwon, M. J., Choi, J., Baek, K., & O’Loughlin, E. J. (2014). The transport behavior of As, Cu, Pb, and Zn during electrokinetic remediation of a contaminated soil using electrolyte conditioning. Chemosphere, 117, 79–86.
Yip, T. C., Tsang, D. C., Ng, K. T., & Lo, I. M. (2009). Empirical modeling of heavy metal extraction by EDDS from single-metal and multi-metal contaminated soils. Chemosphere, 74(2), 301–307.
Yuan, C., & Weng, C. H. (2004). Remediating ethylbenzene-contaminated clayey soil by a surfactant-aided electrokinetic (SAEK) process. Chemosphere, 57(3), 225–232.
Yuan, C., & Weng, C. H. (2006). Electrokinetic enhancement removal of heavy metals from industrial wastewater sludge. Chemosphere, 65(1), 88–96.
Zhou, D. M., Deng, C. F., & Cang, L. (2004). Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents. Chemosphere, 56(3), 265–273.
Funding
None.
Author information
Authors and Affiliations
Contributions
HA contributed to conceptualization. SB, HA, NA, AAJ contributed to methodology. SB, HA helped in writing—original draft preparation. AAJ, NA contributed to writing—review editing. HA, NA, AAJ helped in supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behrouzinia, S., Ahmadi, H., Abbasi, N. et al. Insights into enhanced electrokinetic remediation of copper-contaminated soil using a novel conductive membrane based on nanoparticles. Environ Geochem Health 44, 1015–1032 (2022). https://doi.org/10.1007/s10653-021-01006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01006-w