Abstract
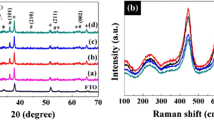
Films of TiO2 nanorods prepared under different conditions of hydrothermal synthesis were studied. With an increase in the hydrothermal synthesis temperature at a synthesis time of 24 h, the length of the TiO2 nanorods formed first increases and then decreases, and the nanorod diameter changes. The TiO2 film consisting of nanorods 4100 nm long and 100 nm in diameter, prepared at 180°C, exhibits the highest photocatalytic activity. This effect is predominantly associated with high specific surface area of the samples.






Similar content being viewed by others
REFERENCES
Lazar, M.A., Varghese, S., and Nair, S.S., Catalysts, 2012, vol. 2, no. 4, pp. 572–601. https://doi.org/10.3390/catal2040572
Zhao, Y., Hoivik, N., and Wang, K.Y., Nano Energy, 2016, vol. 30, pp. 728–744. https://doi.org/10.1016/j.nanoen.2016.09.027
Serikov, T.M., Ibrayev, N.K., Nuraje, N., Savilov, S.V., and Lunin, V.V., Russ. Chem. Bull., 2017, vol. 66, no. 4, pp. 614–621. https://doi.org/10.1007/s11172-017-1781-0
Wang, F.Y., Song, L.F., and Zhang, H.C., J. Electron. Mater., 2017, vol. 46, no. 8, pp. 4716–4724. https://doi.org/10.1007/s11664-017-5491-z
Serikov, T.M., Ibrayev, N.K., and Smagulov, Z., IOP Conf. Ser. Mater. Sci. Eng., 2016, vol. 110, ID 012066. https://doi.org/10.1088/1757-899X/110/1/012066
Yamazaki, Y., Fujitsuka, M., and Yamazaki, S., ACS Appl. Nano Mater., 2019, vol. 2, pp. 5890−5899. https://doi.org/10.1021/acsanm.9b01334
Liu, B., Boercker, J.E., and Aydil, E.S., Nanotechnology, 2008, vol. 19, no. 50, pp. 505604–505609. https://doi.org/10.1088/0957-4484/19/50/505604
Yamazaki, Y., Azami, K., Katoh, R., and Yamazaki, S., ACS Appl. Nano Mater., 2018, vol. 10, pp. 5927–5935. https://doi.org/10.1021/acsanm.8b01617
Hwang, Y.J., Hahn, C., Liu, B., and Yang, P., ACS Nano, 2012, vol. 6, no. 6, pp. 5060–5069. https://doi.org/10.1021/nn300679d
Kerkez, Ö. and Boz, I., React. Kinet. Mech. Catal., 2013, vol. 110, pp. 543–557. https://doi.org/10.1007/s11144-013-0616-8
Liu, B. and Aydil, E.S., J. Am. Chem. Soc., 2009, vol. 131, no. 11, pp. 3985–3990. https://doi.org/10.1021/ja8078972
Ravidhas, C., Anitha, B., Arivukarasan, D., Venkatesh, R., Christy, A.J., Jothivenkatachalam, K., and Sanjeeviraja, C., J. Mater. Sci.: Mater. Electron., 2016, vol. 27, no. 5, pp. 5020–5032. https://doi.org/10.1007/s10854-016-4389-5
Kwon, C.H., Shin, H.M., Kim, J.H., Choi, W.S., and Yoon, K.H., Mater. Chem. Phys., 2004, vol. 86, no. 1, pp. 78–82. https://doi.org/10.1016/j.matchemphys.2004.02.024
Kahr, G. and Madsen, F.T., Appl. Clay Sci., 1995, vol. 9, no. 5, pp. 327–336. https://doi.org/10.1016/0169-1317(94)00028-o
Funding
The study was performed within the framework of grants of the Scientific Committee of the Ministry of Education and Science of the Kazakhstan Republic: Grants for Supporting Postdoc (PhD) Research and Training. Grant Program of Type A, nos. APP-PHD-A-19/004P and AP08052675, and also within the framework of the government assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of basic research and of the state budget theme of the Faculty of Chemistry of the Moscow State University “Catalysis and Physical Chemistry of the Surface” (state registry no. AAAA-A16-116092810057-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 4, pp. 445–452, January, 2021 https://doi.org/10.31857/S0044461821040034
Rights and permissions
About this article
Cite this article
Serikov, T.M., Ibrayev, N.K., Ivanova, T.M. et al. Influence of the Hydrothermal Synthesis Conditions on the Photocatalytic Activity of Titanium Dioxide Nanorods. Russ J Appl Chem 94, 442–449 (2021). https://doi.org/10.1134/S1070427221040030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427221040030