Abstract
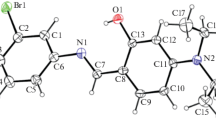
Two crystalline diastereomorphs of rac-1-benzyl-3-bromo-5-hydroxy-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2Н-pyrrole-2-one are obtained and characterized: the racemic compound (P21/c) and the normal conglomerate (P65, P61). The conglomerate is shown to be more thermodynamically preferred while the racemic compound is a metastable form in the entire temperature range studied. These two modifications are formed as a mixture during routine crystallization from a solution and characterized by a similar density, however, their structures significantly differ in the parameters of hydrogen bonds. A small difference in the free energies of two phases at room temperature, which is found by differential scanning calorimetry, explains the experimental availability of both forms during crystallization from the solution. The formation of a stronger hydrogen bond in the conglomerate crystals, which is detected by X-ray diffraction and solid-state vibrational spectroscopy, may be explained by a more favorable arrangement of donor and acceptor groups of the neighboring molecules in a homochiral helix of the conglomerate as compared with a heterochiral dimer, which is the main motif in crystals of the racemic compound.












Similar content being viewed by others
REFERENCES
W. H. Brooks, W. C. Guida, and K. G. Daniel. Curr. Top. Med. Chem., 2011, 11, 760.
Y. Wang and A. M. Chen. Org. Process Res. Dev., 2008, 12, 282.
H. Lorenz and A. Seidel-Morgenstern. Angew. Chem., Int. Ed., 2014, 53, 1218.
A. Collet. In: Comprehensive Supramolecular Chemistry, Vol. 10 / Ed. D. N. Reinhoudt. Pergamon: Oxford, 1996, 113.
E. Pidcock. Chem. Commun., 2005, 27, 3457.
C. Pratt-Brock and J. D. Dunitz. Chem. Mater., 1994, 6, 1118.
R. G. Kostyanovsky, A. P. Avdeenko, S. A. Konovalova, G. K. Kadorkina, and A. V. Prosyanik. Mendeleev Commun., 2000, 10, 16.
J. Jacques, A. Collet, and S. H. Wilen. Enantiomers, Racemates and Resolutions. Krieger Publishing Company, 1994.
C. P. Brock, W. B. Schweizer, and J. D. Dunitd. J. Am. Chem. Soc., 1991, 113, 9811.
Q. He, S. Rohani, J. Zhu, and H. Gomaa. Cryst. Growth Des., 2010, 10, 5136.
P. A. Levkin, Y. A. Strelenko, K. A. Lyssenko, V. Schurigd, and R. G. Kostyanovskya. Tetrahedron: Asymmetry, 2003, 14, 2059.
V. N. Khrustalev, B. Sandhu, S. Bentum, A. Fonari, A. V. Krivoshein, and T. V. Timofeeva. Cryst. Growth Des., 2014, 14, 3360.
O. A. Lodochnikova, Y. K. Voronina, L. Z. Latypova, D. B. Krivolapov, A. R. Kurbangalieva, and I. A. Litvinov. Russ. Chem. Bull., 2013, 62, 1218.
O. A. Lodochnikova, L. S. Kosolapova, A. F. Saifina, A. T. Gubaidullin, R. R. Fayzullin, A. R. Khamatgalimov,
O. A. Lodochnikova, A. R. Zaripova, R. R. Fayzullin, A. I. Samigullina, I. I. Vandyukova, L. N. Potapova, and A. R. Kurbangalieva. CrystEngComm, 2018, 20, 3218.
D. P. Gerasimova, A. F. Saifina, D. V. Zakharychev, I. I. Vandyukova, R. R. Fayzullin, A. R. Kurbangalieva, and
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. J. Appl. Crystallogr., 2015, 48, 3.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3.
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3.
S. Parsons, H. D. Flack, and T. Wagner. Acta Crystallogr., Sect. B: Struct. Sci. Cryst. Eng. Mater., 2013, 69, 249.
L. J. Farrugia. J. Appl. Crystallogr., 2012, 45, 849.
R. R. Fayzullin, D. V. Zakharychev, A. T. Gubaidullin, O. A. Antonovich, D. B. Krivolapov, Z. A. Bredikhina, and A. A. Bredikhin. Cryst. Growth Des., 2017, 17, 271.
S. A. Shteingolts, V. V. Davydova, M. A. Maryasov, O. E. Nasakin, R. R. Fayzullin, and O. A. Lodochnikova. J. Struct. Chem., 2020, 61(6), 928.
S. A. Shteingolts, A. F. Saifina, L. F. Saifina, V. E. Semenov, G. K. Fukin, and R. R. Fayzullin. J. Mol. Struct., 2021, 1228, 129724.
R. Taylor, O. Kennard, and W. Versichel. J. Am. Chem. Soc., 1983, 105, 5761.
A. Vedani and J. D. Dunitz. J. Am. Chem. Soc., 1985, 107, 7653.
M. Ahmed, C. Jelsch, B. Guillot, C. Lecomte, and S. Domagala. Cryst. Growth Des., 2012, 13, 315.
Funding
The work was supported by Russian Science Foundation grant No. 17-13-01209.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 5, pp. 781-794.https://doi.org/10.26902/JSC_id72892
Rights and permissions
About this article
Cite this article
Gerasimova, D.P., Saifina, A.F., Zakharychev, D.V. et al. CHIRALITY-DEPENDENT HYDROGEN BONDING AND ENERGY OF 1-BENZYL-3-BROMO-5-HYDROXY- 4-[(4-METHYLPHENYL)SULFANYL]-1,5-DIHYDRO- 2Н-PYRROLE-2-ONE DIASTEREOMORPHS. J Struct Chem 62, 727–739 (2021). https://doi.org/10.1134/S0022476621050097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621050097