Abstract
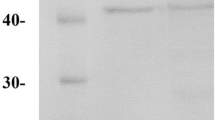
The extracellular enzyme with oxidase function was extracted from the Neonothopanus nambi luminescent fungus by using mild processing of mycelium with β-glucosidase and then isolated by gel-filtration chromatography. The extracted enzyme is found to be a FAD-containing protein, catalyzing phenol co-oxidation with 4-aminoantipyrine without addition of H2O2, which distinguishes it from peroxidases. This fact allowed us to assume that this enzyme may be a mixed-function oxidase. According to gel-filtration chromatography and SDS-PAGE, the oxidase has molecular weight of 60 kDa. The enzyme exhibits maximum activity at 55–70 °C and pH 5.0. Kinetic parameters Km and Vmax of the oxidase for phenol were 0.21 mM and 0.40 µM min−1. We suggest that the extracted enzyme can be useful to develop a simplified biosensor for colorimetric detection of phenol in aqueous media, which does not require using hydrogen peroxide.










Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 4-AAP:
-
4-Aminoantipyrine (1-phenyl-2,3-dimethyl-4-aminopyrazolone)
- BSA:
-
Bovine serum albumin
- DI:
-
Deionized water
- FAD:
-
Flavin adenine dinucleotide
- SDS-PAGE:
-
Sodium dodecyl sulfate- polyacrylamide gel electrophoresis
References
Wong DWS (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Knop D, Yarden O, Hadar Y (2015) The ligninolytic peroxidases in the genus Pleurotus: divergence in activities, expression, and potential applications. Appl Microbiol Biotechnol 99:1025–1038
Sützl L, Laurent CVFP, Abrera AT, Schütz G, Ludwig R, Haltrich D (2018) Multiplicity of enzymatic functions in the CAZy AA3 family. Appl Microbiol Biotechnol 102:2477–2492
Daniel G, Nilsson T, Pettersson B (1989) Intra- and extracellular localization of lignin peroxidase during the degradation of solid wood and wood fragments by Phanerochaete chrysosporium by using transmission electron microscopy and immuno-gold labeling. Appl Environ Microbiol 55:871–881
van den Brink J, de Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1492
Hernández-Ortega A, Ferreira P, Martínez AT (2012) Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol 93:1395–1410
Wongnate T, Chaiyen P (2013) The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily. FEBS J 280:3009–3027
Ai M-Q, Wang F-F, Zhang Y-Z, Huang F (2014) Purification of pyranose oxidase from the white rot fungus Irpex lacteus and its cooperation with laccase in lignin degradation. Process Biochem 49:2191–2198
Qiu H, Li Y, Ji G, Zhou G, Huang X, Qu Y, Gao P (2009) Immobilization of lignin peroxidase on nanoporous gold: enzymatic properties and in situ release of H2O2 by co-immobilized glucose oxidase. Bioresour Technol 100:3837–3842
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Martínková L, Kotik M, Marková E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere 149:373–382
Galperin I, Javeed A, Luig H, Lochnit G, Rühl M (2016) An aryl-alcohol oxidase of Pleurotus sapidus: heterologous expression, characterization, and application in a 2-enzyme system. Appl Microbiol Biotechnol 100:8021–8030
Martínez AT, Ruiz-Dueñas FJ, Camarero S et al (2017) Oxidoreductases on their way to industrial biotransformations. Biotechnol Adv 35:815–831
Li F, Ma W, Wu X, Wang Y, He J (2018) Luminol, horseradish peroxidase, and glucose oxidase ternary functionalized graphene oxide for ultrasensitive glucose sensing. Anal Bioanal Chem 410:543–552
Şahin S, Wongnate T, Chuaboon L, Chaiyen P, Yu E (2018) Enzymatic fuel cells with an oxygen resistant variant of pyranose-2-oxidase as anode biocatalyst. Biosens Bioelectron 107:17–25
Dubey MK, Zehra A, Aamir M, Meena M, Ahirwal L, Singh S, Shukla S, Upadhyay RS, Bueno-Mari R, Bajpai VK (2017) Improvement strategies, cost effective production, and potential applications of fungal glucose oxidase (GOD): current updates. Front Microbiol 8:1032
Sarma R, Islam M, Running M, Bhattacharyya D (2018) Multienzyme immobilized polymeric membrane reactor for the transformation of a lignin model compound. Polymers 10:463
Savino S, Fraaije MW (2020) The vast repertoire of carbohydrate oxidases: an overview. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2020.107634
Ferreira P, Medina M, Guillén F, Martínez M, van Berkel W, Martínez A (2005) Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem J 389:731–738
Tamaru Y, Umezawa K, Yoshida M (2018) Characterization of an aryl-alcohol oxidase from the plant saprophytic basidiomycete Coprinopsis cinerea with broad substrate specificity against aromatic alcohols. Biotechnol Lett 40:1077–1086
Jankowski N, Koschorreck K, Urlacher VB (2020) High-level expression of aryl-alcohol oxidase 2 from Pleurotus eryngii in Pichia pastoris for production of fragrances and bioactive precursors. Appl Microbiol Biotechnol 104:9205–9218
Goswami P, Chinnadayyala SS, Chakraborty M, Kumar AK, Kakoti A (2013) An overview on alcohol oxidases and their potential applications. Appl Microbiol Biotechnol 97:4259–4275
Romero E, Ferreira P, Martínez Á, Martínez M (2009) New oxidase from Bjerkandera arthroconidial anamorph that oxidizes both phenolic and nonphenolic benzyl alcohols. Biochim Biophys Acta 1794:689–697
Caves MS, Derham BK, Jezek J, Freedman RB (2011) The mechanism of inactivation of glucose oxidase from Penicillium amagasakiense under ambient storage conditions. Enzyme Microb Technol 49:79–87
Glazunova OA, Shakhova NV, Psurtseva NV, Moiseenko KV, Kleimenov SY, Fedorova TV (2018) White-rot basidiomycetes Junghuhnia nitida and Steccherinum bourdotii: Oxidative potential and laccase properties in comparison with Trametes hirsuta and Coriolopsis caperata. PLoS ONE 13(6):e0197667. https://doi.org/10.1371/journal.pone.0197667
Lueangjaroenkit P, Teerapatsakul C, Sakka K, Sakka M, Kimura T, Kunitake E, Chitradon L (2019) Two Manganese Peroxidases and a Laccase of Trametes polyzona KU-RNW027 with Novel Properties for Dye and Pharmaceutical Product Degradation in Redox Mediator-Free System. Mycobiology 47:217–229
Mogilnaya OA, Ronzhin NO, Posokhina ED, Bondar VS (2020) Production of extracellular oxidases in the mycelium of the bioluminescent Neonothopanus nambi (Omphalotaceae, Basidiomycota) grown in submerged culture in different media. Asian J Mycol 3(1):408–418
Vina-Gonzalez J, Elbl K, Ponte X, Valero F, Alcalde M (2018) Functional expression of aryl-alcohol oxidase in Saccharomyces cerevisiae and Pichia pastoris by directed evolution. Biotechnol Bioeng 115:1666–1674
Tu T, Wang Y, Huang H, Wang Y, Jiang X, Wang Z, Yao B, Luo H (2019) Improving the thermostability and catalytic efficiency of glucose oxidase from Aspergillus niger by molecular evolution. Food Chem 281:163–170
Ghoshdastider U, Wu R, Trzaskowski B et al (2015) Molecular effects of encapsulation of glucose oxidase dimer by graphene. RSC Adv 5:13570–13578
Giannakopoulou A, Patila M, Spyrou K et al (2019) Development of a four-enzyme magnetic nanobiocatalyst for multi-step cascade reactions. Catalysts 9:995
Koenig M, König U, Eichhorn K, Müller M, Stamm M, Uhlmann P (2019) In-situ-investigation of enzyme immobilization on polymer brushes. Front Chem 7:101
Mogilnaya OA, Ronzhin NO, Bondar VS (2018) Estimating levels of light emission and extracellular peroxidase activity of mycelium of luminous fungus Neonothopanus nambi treated with β-glucosidase. Curr Res Environ Appl Mycol 8:75–85
Mogilnaya OA, Ronzhin NO, Artemenko KS, Bondar VS (2018) Creation of bifunctional indicating complex based on nanodiamonds and extracellular oxidases of luminous fungus Neonothopanus nambi. Dokl Biochem Biophys 480:135–138
Mogilnaya O, Ronzhin N, Artemenko K, Bondar VS (2019) Nanodiamonds as an effective adsorbent for immobilization of extracellular peroxidases from luminous fungus Neonothopanus nambi to construct a phenol detection system. Biocatal Biotransform 37:97–105
Mogilnaya OA, Ronzhin NO, Artemenko KS, Posokhina ED, Bondar VS (2020) Extracellular oxidases of basidiomycete Neonothopanus nambi: isolation and some properties. Dokl Biochem Biophys 490:38–42
Kochetov GA (1980) Practical guide to enzymology. Higher School, Moscow ((in Russian))
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Asada Y, Watanabe A, Ohtsu Y, Kuwahara M (1995) Purification and characterization of an aryl-alcohol oxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Biosci Biotechnol Biochem 59:1339–1341
Funding
This work was supported by the state budget allocated to the fundamental research at the Russian Academy of Sciences, Project No. 0356–2019-0022.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions in conceptualizing, drafting, developing and reviewing the manuscript. The paper was reviewed and approved by all authors prior to submission for peer review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mogilnaya, O., Ronzhin, N., Posokhina, E. et al. Extracellular Oxidase from the Neonothopanus nambi Fungus as a Promising Enzyme for Analytical Applications. Protein J 40, 731–740 (2021). https://doi.org/10.1007/s10930-021-10010-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-021-10010-z