Abstract
Mitochondrial morphology varies according to development and the physiological conditions of the cell. Here, we performed electron tomography using serial sections to analyze the number, individual volume, and morphological complexity of mitochondria in the cells across two generations in the life cycle of the brown alga Mutimo cylindricus. This species shows a heteromorphic alternation of generations between the macroscopic gametophyte and the crustose sporophyte during its life cycle and displays anisogamous sexual reproduction. We observed the mitochondria in the vegetative cells of gametophytes and sporophytes to mainly show tubular or discoidal shapes with high morphological complexity. The morphology of the mitochondria in the male and female gametes changed to a nearly spherical or oval shape from a tubular or discoidal shape before release. In this species, degradation of the paternal mitochondria was observed in the zygote 2 h after fertilization. Morphological changes in the mitochondria were not observed until 6 h after fertilization. Twenty-four-hour-old zygotes before and after cytokinesis showed a similar number of mitochondria as 6-h-old zygotes; however, the volume and morphological complexity increased. The results indicated that the maternal mitochondria did not undergo fission or fusion until this stage. Based on the analysis results of the number and total volume of mitochondria before and after the release of the gametes, it is possible that the mitochondria in the female gametes fuse immediately before release.







Similar content being viewed by others
References
Aoyama H, Kuroiwa T, Nakamura S (2009) The dynamic behaviour of mitochondria in living zygotes during maturation and meiosis in Chlamydomonas reinhardtii. Eur J Phycol 44:497–507
Berkaloff C, Rousseau B (1979) Ultrastructure of male gametogensis in Fucus serratus (Phaeophycae). J Phycol 15:163–173
Brawley SH, Wetherbee R, Quatrano RS (1976) Fine–structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). II. The cytoplasm of the egg and young zygote. J Cell Sci 20:255–271
Chan DC (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46:265–287
Clayton MN (1984) An electron microscope study of gamete release and settling in the complanate from Scytosiphon (Scytosiphonaceae, Phaeophyta). J Phycol 20:276–285
Clayton MN (1986) Culture studies on the life history of Scytothamnus australis and Scytothamnus fasciculatus (Phaeophyta) with electron microscope observations on sporogenesis and gametogenesis. Br Phycol J 21:371–386
Cui Y, Cao W, He Y, Zhao Q, Wakazaki M, Zhuang X, Gao J, Zeng Y, Gao C, Ding Y, Wong HY, Wong WS, Lam HK, Wang P, Ueda T, Rojas-Pierce M, Toyooka K, Kang BH, Jiang L (2019) A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat Plants 5:95–105
De Martino C, Floridi A, Marcante ML, Malorni W, Scorza Barcellona P, Bellocci M, Silvestrini B (1979) Morphological, histochemical and biochemical studies on germ cell mitochondria of normal rats. Cell Tissue Res 196:1–22
Ehara T, Osafune T, Hase E (1995) Behavior of mitochondria in synchronized cells of Chlamydomonas reinhardtii (Chlorophyta). J Cell Sci 108:499–507
Fu G, Nagasato C, Ito T, Müller DG, Motomura T (2013) Ultrastructural analysis of flagellar development in plurilocular sporangia of Ectocarpus siliculosus (Phaeophyceae). Protoplasma 250:261–272
Hales KG, Fuller MT (1997) Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90:121–129
Henry EC, Cole KM (1982a) Ultrastructure of swarmers in the Laminariales (Phaeophyceae). I. Zoospores. J Phycol 18:550–569
Henry EC, Cole KM (1982b) Ultrastructure of swarmers in the Laminariales (Phaeophyceae). II. Sperm. J Phycol 18:570–579
Ho HC, Wey S (2007) Three dimensional rendering of the mitochondrial sheath morphogenesis during mouse spermiogenesis. Microsc Res Tech 70:719–723
Katsaros C, Galatis B (1986) Ultrastructural studies on zoosporogenesis of Halopteris filicina (Sphacelariales, Phaeophyta). Phycologia 25:358–370
Kawai H, Kogishi K, Hanyuda T, Kitayama T (2012) Taxonomic revision of the genus Cutleria proposing a new genus Mutimo to accommodate M. cylindricus (Cutleriaceae, Phaeophyceae). Psychol Res 60:241–248
Kinoshita N, Fu G, Ito T, Motomura T (2016) Three-dimensional organization of flagellar basal apparatus in Ectocarpus gametes. Phycol Res 64:19–25
Kitayama T, Kawai H, Yoshida T (1992) Dominance of female gametophytes in field populations of Cutleria cylindrica (Cutleriales, Phaeophyceae) in the Tsugaru Strait, Japan. Phycologia 31:449–461
Kremer JR, Mastronarde DN, McIntosh JR (1996) Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116:71–76
Kuroiwa T (2010) Mechanisms of organelle division and inheritance and their implications regarding the origin of eukaryotic cells. Proc Jpn Acad Ser B Phys Biol Sci 86:455–471
La Claire JW, West JA (1978) Light-and electron-microscopic studies of growth and reproduction in Cutleria (Phaeophyta). Protoplasma 97:93–110
La Claire JW, West JA (1979) Light–and electron–microscopic studies of growth and reproduction in Cutleria (phaeophyta). Protoplasma 101:247–267
Lowe DG (2004) Distinctive image features from scale-invariant keypoints. Int J Comput Vis 60:91–110
Maier I (1997) The fine structure of the male gamete of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae). I. General structure of the cell. Eur J Phycol 32:241–253
Markey DR, Wilce RT (1975) The ultrastructure of reproduction in the brown alga Pylaiella littoralis. I. Mitosis and cytokinesis in the plurilocular gametangia. Protoplasma 85:219–241
Murtin C, Frindel C, Rousseau D, Ito K (2018) Image processing for precise three-dimensional registration and stitching of thick high-resolution laser-scanning microscopy image stacks. Comput Biol Med 92:22–41
Nagasato C, Kajimura N, Terauchi M, Mineyuki Y, Motomura T (2014) Electron tomographic analysis of cytokinesis in the brown alga Silvetia babingtonii (Fucales, Phaeophyceae). Protoplasma 251:1347–1357
Nagasato C, Motomura T (2002) Ultrastructural study on mitosis and cytokinesis in Scytosiphon lomentaria zygotes (Scytosiphonales, Phaeophyceae) by freeze-substitution. Protoplasma 219(3–4):140–149
Nicander L, Ploen L (1969) Fine structure of spermatogonia and primary spermatocytes in rabbits. Z Zellforsch Mikrosk Anat 99:221–234
Peachey LD (1958) Thin sections: I. A study of section thickness and physical distortion produced during microtomy. J Biophys Biochem Cytol 4:233–242
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Culture and collections of algae (Proc Jap Conf Hakone, 1966). Japanese Society of Plant Physiology, Tokyo, pp 63–75
Rantanen A, Larsson NG (2000) Regulation of mitochondrial DNA copy number during spermatogenesis. Hum Reprod 2:86–91
Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114:867–874
Sato M, Sato K (2013) Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim Biophys Acta 1833:1979–1984
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open–source platform for biological-image analysis. Nat Methods 28:676–682
Shen Y, Iwao T, Motomura T, Nagasato C (2020) Cytoplasmic inheritance of mitochondria and chloroplasts in the anisogamous brown alga Mutimo cylindricus. Protoplasma:1–14
Terauchi M, Nagasato C, Kajimura N, Mineyuki Y, Okuda K, Katsaros C, Motomura T (2012) Ultrastructural study of plasmodesmata in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae). Planta 236:1013–1126
Varuzhanyan G, Chan DC (2020) Mitochondrial dynamics during spermatogenesis. J Cell Sci 133:14
Varuzhanyan G, Rojansky R, Sweredoski MJ, Graham RL, Hess S, Ladinsky MS, Chan DC (2019) Mitochondrial fusion is required for spermatogonial differentiation and meiosis. elife 8:e51601
Vincent AE, White K, Davey T, Philips J, Ogden RT, Lawless C, Warren C, Hall MG, Ng YS, Falkous G, Holden T, Deehan D, Taylor RW, Turnbull DM, Picard M (2019) Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep 26:996–1009
Yoshida Y, Kuroiwa H, Shimada T, Masaki Yoshida M, Mio Ohnuma M, Takayuki Fujiwara T, Yuuta Imoto Y, Fumi Yagisawa F, Keiji Nishida K, Shunsuke Hirooka S, Osami Misumi O, Yuko Mogi Y, Yoshihiko Akakabe Y, Kazunobu Matsushita K, Tsuneyoshi Kuroiwa T (2017) Glycosyltransferase MDR1 assembles a dividing ring for mitochondrial proliferation comprising polyglucan nanofilaments. Proc Natl Acad Sci U S A 114:13284–13289
Acknowledgements
We are grateful to Dr. Toyoki Iwao (Toba Fisheries Science Center) for collecting the fresh Mie-strain of M. cylindricus, and to Dr. Toshiaki Ito (Electron Microscope Laboratory, Research Faculty of Agriculture, Hokkaido University) for preparing the TEM samples of the gametophyte stage using high-pressure freezing.
Funding
This work was supported by the Sumitomo Foundation (grant number 190311).
Author information
Authors and Affiliations
Contributions
Yuan Shen analyzed the data and maintained the M. cylindricus strains. Chikako Nagasato and Taizo Motomura designed the experiments and critically reviewed the manuscript. All authors have written and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Handling Editor: Tsuneyoshi Kuroiwa
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Fig. S1
Serial ultrathin sections used for 3D reconstruction a Ribbon of thin serial sections generated using a diamond knife. b Enlarged image of a with interference color as gold. (PNG 992 kb)
Fig. S2
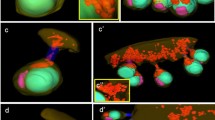
Three-dimensional models used for quantitative analysis of mitochondrial morphology across gametophyte-to-sporophyte life cycle of M. cylindricus. All mitochondria used for quantitative analysis originated from Table S1. a1–3 Male_Gametophyte_1–3. b1–3 Female_Gametophyte_1–3. c1–3 Male_Gametangium_1–3. d1–3 Female_Gametangium_1–3. e1–3 Male_Gamete_1–3. F1–3 Female_Gamete_1–3. g1–3 2-h-old_Zygote_1–3. h1–3 6-h-old_Zygote_1–3. i1–2 24-h-old_Zygote_1 to 2. k1–4: 2-celled_Sporophyte_1–2. l1–3 20-d-old_Sporophyte_1 to 3. The plasma membrane is indicated in gray. Nuclei are indicated in blue. Mitochondria are indicated in orange. Scale bar: 1 μm. (PNG 3233 kb)
Fig. S3
Gametophyte-to-sporophyte life cycle of M. cylindricus a, b Male and female gametophytes. Arrowheads indicate released swimming gametes. c, d Male and female plurilocular gametangia. Black arrowheads indicate orange eyespots in gametangia small loci. White arrowheads indicate somatic cells of gametophytes. e, f Released swimming male and female gametes with one eyespot (arrowhead) in each cell. g Zygote with two orange eyespots (arrowheads). h Two-celled sporophytes with two orange eyespots (arrowheads). i Crustose sporophyte. af, anterior flagellum; pf, posterior flagellum. Scale bar: 1 cm in a, b; 10 μm in c–h. (PNG 4617 kb)
Table S1
MPG, Male_Gametangium; FPG, Female_Gametangium; Mg, Male_Gamete; Fg, Female_Gamete; 2hZ, 2-h-old_Zygote; 6hZ, 6-h-old_Zygote; 24hZ, 24-h-old_Zygote; 24h2cS, 2-celled_Sporophyte; 20dS, 20-d-old_Sporophyte. Within the box plot, the solid line represents the 50th percentile, the dashed line represents the mean, the box delimits the 25th and 75th percentiles, and bars indicate the 10th and 90th percentiles. Within the line plot, data are mean ± SEM (DOCX 40 kb)
Movie S1
A 3D model of a male gametophyte (MOV 2559 kb)
Movie S2
A 3D model of a female gametophyte (MOV 3778 kb)
Movie S3
A 3D model of a male gametangium (MOV 2311 kb)
Movie S4
A 3D model of a female gametangium (MOV 3856 kb)
Movie S5
A 3D model of a male gamete (MOV 628 kb)
Movie S6
A 3D model of a female gamete (MOV 2662 kb)
Movie S7
A 3D model of a 2-h-old zygote (MOV 2586 kb)
Movie S8
A 3D model of a 6-h-old zygote (MOV 3305 kb)
Movie S9
A 3D model of a 24-h-old zygote (MOV 2595 kb)
Movie S10
A 3D model of a two-celled sporophyte (MOV 2842 kb)
Movie S11
A 3D model of the marginal apical region in a 20-d-old sporophyte (MOV 5054 kb)
Movie S12
A 3D model of the subapical region in a 20-d-old sporophyte (MOV 3460 kb)
Movie S13
A 3D model of the central region in a 20-d-old sporophyte (MOV 7639 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Motomura, T. & Nagasato, C. Ultrastructural observations of mitochondrial morphology through the life cycle of the brown alga, Mutiomo cylindricus (Cutleriaceae, Tilopteridales). Protoplasma 259, 371–383 (2022). https://doi.org/10.1007/s00709-021-01679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01679-1