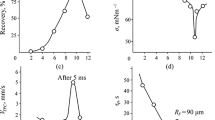
Abstract
The action of the arbitrary physisorbed species of a collector at a mineral is compared with the theoretical evidence on particle–bubble attachment. The lack of the correlation between the hydrophobic behavior, characterized by the wetting angle, and the floatability of minerals, as well as the correlation between the inductance time and the mineral recovery are discussed. The causes of disagreement between the collectability sequence of xanthates, dithiophosphates and dithiocarbamates and the sequence of boost in energy of chemical bond between these reagents and cation of mineral lattice are exposed. Collectabilities of frothers and residues of the collectors are explained. The ways to increase flotation performance are shown, namely, the mineral recovery and the concentrate quality can be improved by means of adjustment of the chemisorbable /physisorbable collector ratio.



Similar content being viewed by others
REFERENCES
Abramov, A.A., Principles of Selection and Synthesis of Higher Selective Collectors in Flotation, Tsvet. Metally, 2009, No. 4, pp. 35–40.
Kondrat’ev, S.A., Physically Sorbed Collectors in Froth Flotation and Their Activity. Part I, Journal of Mining Science, 2008, vol. 44, no. 6, pp. 628–635.
Kondrat’ev, S.A., Physically Sorbed Collectors in Froth Flotation and Their Activity. Part II, Journal of Mining Science, 2009, vol. 45, no. 2, pp. 173–181.
Kondrat’ev, S.A. and Moshkin, N.P., Foam Separation Selectivity Conditioned by the Chemically Attached Agent, Journal of Mining Science, 2014, vol. 50, no. 4, pp. 780–787.
Kondrat’ev, S.A. and Moshkin, N.P., Estimate of Collecting Force of Flotation Agent, Journal of Mining Science, 2015, vol. 51, no. 1, pp. 150–156.
Kondrat’ev, S.A., Moshkin, N.P., and Konovalov, I.A., Collecting Ability of Easily Desorbed Xanthates, Journal of Mining Science, 2015, vol. 51, no. 4, pp. 830–838.
Kondrat’ev, S.A., Moshkin, N.P., and Burdakova, E.A., Determination of Activity/Selectivity Ratio in Chemical and Physical Adsorption of a Reagent, Journal of Mining Science, 2016, vol. 52, no. 5, pp. 965–973.
Sutherland, K.L. and Wark, I.W., Principles of Flotation, Principles of Flotation, Austr. Inst. Min. Metall., Melbourne, Australia, 1955.
Klassen, V.I. and Tikhonov, S.A., Effect of Sodium Oleate on Flotation Properties of Surface of Air Bubbles, Tsvet. Metally, 1960, no. 10, pp. 4–8.
Finch, J.A. and Smith, G.W., Bubble–Solid Attachment as a Function of Bubble Surface Tension, Can. Metall. Q., 1975, vol. 14, issue 1, pp. 47–51.
Gardner, J.R. and Woods, R., An Electrochemical Investigation of the Natural Floatability of Chalcopyrite, Int. J. Miner. Process., 1979, vol. 6, pp. 1–16.
Babel, B. and Rudolph, M., Investigating Reagent–Mineral Interactions by Colloidal Probe Atomic Force Microscopy, XXIV Int. Miner. Process. Congress Proceedings, Moscow, 2018, pp. 1384–1391.
Kelebek, S., Finch, J.A., Yörük, S., and Smith, G.W., Wettability and Floatability of Galena–Xanthate System as a Function of Solution Surface Tension, Colloids Surf., 1986, vol. 20, pp. 89–100.
Dolzhenkova, A.N. and Kholodnitsky, B.A., Measurement of Wetting Angles: A Case-Study of Flotation, Obog. Rud, 1975, no. 5, pp. 40–43.
Laskowski, J.S., Thermodynamic and Kinetic Flotation Criteria,Miner. Process. Extr. Metall. Rev., 1989, 5, pp. 25–41.
Kloppers, L., Maree, W., Oyekola, O., and Hangone, G., Froth Flotation of Merensky Reef Platinum-Bearing Ore Using Mixtures of SIBX with a Dithiophosphate and a Dithiocarbamate, Miner. Eng., 2015, vol. 20, pp. 1047–1053.
Karimian, A., Rezaei B., and Masoumi A. The Effect of Mixed Collectors in the Rougher Flotation of Subgun Copper, Life Sci. J., 2013, vol. 10, pp. 268–272.
McFadzean, B. and Castelyn, D.G., and O’Connor, C.T., The Effect of Mixed Thiol Collectors on the Flotation of Galena, Miner. Eng., 2012, vol. 36–38, pp. 211–218.
Bagci, E., Ekmekci, Z., and Bradshow, D.J., Adsorption Behavior of Xanthate and Dithiophosphinate from Their Mixtures on Chalcopyrite,Miner. Eng., 2007, vol. 20, pp. 1047–1053.
Nagaraj, D.R. and Ravishankar, S.A., Flotation Reagents—A Critical Overview from an Industry Perspective, Froth Flotation: A Century of Innovation, Fuerstenau M.C., Graeme J., Yoon R.H. (Eds.), Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, 2007.
Bradshaw D.J., Synergistic Effects between Thiol Collectors Used in the Flotation of Pyrite Ph.D. Thesis University of Cape Town 1997.
Lotter N.O. and Bradshaw D.J., The Formulation and Use of Mixed Collectors in Sulphide Flotation Miner. Eng., 2010, vol. 23, pp. 945–951.
Hangone, G., Bradshaw, D., and Ekmekci, Z., Flotation of Copper Sulphide Ore from Okiep Using Thiol Collectors and Their Mixture,J. S. Afr. Inst. Min. Metall., 2005, vol. 105, pp. 199–206.
Bogdanov, O.S. (Ed.), Teoriya i tekhnologiya flotatsii rud (Theory and Technology of Ore Flotation), Moscow: Nedra, 1980.
Abramov, A.A., Standards of Selection and Construction of Selective Collecting Agents. Part I: Theoretical Framework for the Selection of Selective Collectors, Tsvet. Metally, 20212, no. 4, pp. 17–20.
Taguta J. and O’Connor C. T. McFadzean B., Relating Enthalpies of Adsorption of Thiol Collectors and Collector Mixtures on Base Metal Sulfide Minerals to Their Floatability, The 28th Int. Min. Proc. Congress Proceedings, Canadian Institute of Mining, Metallurgy and Petroleum, 2016. ISBN: 978-1-926872-29-2.
Glembotsky, V.A. and Klassen, V.I., Flotatsionnye metody obogashcheniya (Flotation Methods of Mineral Processing), Moscow: Nedra, 1981.
Goden, A.M., Osnovy obogashcheniya poleznykh iskopaemykh (Fundamentals of Mineral Processing), Moscow: Gosgortekhizdat, 1946.
Melik-Gaikazyan, V.I., Wetting Angles and Applications in Flotation Research, Obog. Rud, 1976, no. 5, pp. 13–20.
Melik-Gaikazyan, V.I. and Voronchikhina, V.V., Identification of Causes of the Apparent Violation of the Young Law,Elektrokhimiya, 1969, vol. 3, no. 4, pp. 418–425.
Malysa, K., Barzyk, W., and Pomianowski, A., Influence of Frothers on Floatability in Flotation of Single Minerals (Quartz and Synthetic Chalcocite), Int. J. Miner. Process., 1981, vol. 8, pp. 329–343.
Heyes, G.W. and Trahar, W.J., The Natural Floatability of Chalcopyrite, Int. J. Miner. Process., 1977, vol. 4, pp. 317–344.
Taggart, A.F., del Guidice, G.M.M., and Ziehl, O.A., The Case for the Chemical Theory of Flotation, Trans. Am. Inst. Min. Metall. Eng., 1934, vol. 112, pp. 348–381.
Dzienisiewies, J. and Pryor, E.J., An Investigation into the Action of Air in Froth Flotation, Bull. Inst. Min. Metall., 1950, vol. 521, pp. 1–23.
Lekki, J. and Laskowski, J.S., On the Dynamic Effect of Frother–Collector Joint Action in Flotation, Transactions IMM, Sec. C, 1971, vol. 80, pp. 174–180.
Derjaguin, B.V. and Dukhin, S.S., Transactions IMM, 1961, vol. 70, P. 221.
Leja, J. and He, Q., The role of Flotation Frothers in the Particle–Bubble Attachment Process, Principles of Mineral Flotation: Symposium Proceedings, M.H. Jones and J.T. Woodcock (Eds.), Austr. Inst. Min. Metall., 1984, pp. 215–232.
Critchley, J.K. and Riaz, M., Study of Synergism between Xanthate and Dithiocarbamate Collectors in Flotation of Heazlewoodite,Trans. Inst. Min. Metall., 1991, vol. 100, pp. 55–57.
Plaksin, I.N. and Zaitseva, S.P., Nacuh. Soobshch. IGD Skochinskogo. Akad. Nauk SSSR, 1960, no. 6, pp. 15–20.
Lotter, N.O., Bradshaw, D.J., and Barnes, A.R., Classification of the Major Copper Sulphides into Semiconductor Types, and Associated Flotation Characteristics, Miner. Eng., 2016, vol. 96–97, pp. 177–184.
Lucassen-Reynders, E.H., Lucassen, J., and Giles, D., Surface and Bulk Properties of Mixed Anionic, Cationic Surfactant Systems,J. Colloid Interface Sci., 1981, vol. 81, no. 1, pp. 150–157.
Jost, F., Leiter, H., and Schwuger, M.J., Synergisms in Binary Surfactant Mixtures, Colloid Poly. Sci., 1988, vol. 266, pp. 554–561.
Sis, H. and Chander, S., Improving Froth Characteristics and Flotation Recovery of Phosphate Ores with Nonionic Surfactants,Miner. Eng., 2003, vol. 16, pp. 587–595.
Lynch, A.J., Johnson, N.W., Manlapig, E.V., and Thorne, C.G.,Mineral and Coal Flotation Circuits-Their Simulation and Control, Amsterdam: Elsevier, 1981.
Von Rybinski, W. and Schwuger, M.J., Adsorption of Surfactant Mixtures in Froth Flotation, Langmuir, 1986, vol. 2, pp. 639–643.
Konovalov, I.A and Kondrat’ev, S.A., Flotation Activity of Xanthogenates, Journal of Mining Science, 2020, vol. 56, no. 1, pp. 104–112.
Plaksin, N.I., Modern Trends in Research into Selective Flotation of Nonferrous and Rare Metals, Sovremennoe sostoyanie i zadachi selektivnoi flotatsii rud (Selective Ore Flotation: Current Situation and Challenges), Moscow: Nauka, 1967.
Vigdergauz, V.E., Prospects of Reducing Molybdenum Loss with Flotation Fines, Advanced Processing Methods and Deep Conversion Technologies for Nonferrous, Rare and Platinum Metals. Plaksin’s Lectures–2006: Conference Proceedings, Krasnoyarsk: 2006, pp. 72–74.
Ngobeni, W.A and Hangone, G., The Effect of Using Pure Thiol Collectors on the Froth Flotation on Pentlandite Containing Ore,South African J. Chem. Eng., 2013, vol. 18 (1), pp. 41–50.
Lipets, M.E., Hydrophobization Mechanism of Ionic Collectors in Flotation, Tsvet. Metally, 1945, no. 5, pp. 42–46.
Bogdanov, O.S., Podnek, A.K., Khanmain, V.Ya., and Yanis, N.A., Theory and Technology of Flotation, Trudy Inst. Mekhanobr, 1959, issue 124.
Leja, J., Surface Chemistry of Froth Flotation, Plenum Press, 1st Edition, New York and London, 1982.
Rao, S.R. and Finch, J.A., Base Metal Oxide Flotation Using Long Chain Xanthates, Int. J. Miner. Process., 2003, vol. 69, pp. 251–258.
Fuerstenau, M.C., Clifford, K.L., and Kuhn, M.C., The Role of Zinc–Xanthate Precipitation in Sphalerite Flotation, Int. J. Miner. Process., 1974, vol. 1, pp. 307–318.
Chanturiya, V. and Kondratiev, S., Contemporary Understanding and Developments in the Flotation Theory of Non-Ferrous Ores,Miner. Process. Extr. Metall. Rev., 2019, vol. 40, issue 6, pp. 390–401.
Aleinikov, N.A., Nikishin, G.I., Ogibin, Yu.P., and Petrov, A.D., Flotation Properties of Branched Carboxylic Aids, Zh. Prikl. Khim., 1962, vol. 35, no. 9, pp. 2078–2085.
Rosen, M.J., The Relationship of Structure to Properties in Surfactants. IV. Effectiveness in Surface or Inter-Facial Tension Reduction, J. Colloid and Interface Sci., 1976, vol. 56, no. 2, pp. 320–327.
Rosen, M.J., Surfactants and Interfacial Phenomena, Chapter 5: Reduction of Surface and Interfacial Tension by Surfactants, John Wiley & Sons, Inc., Hoboken, 2004, pp. 208–242.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fiziko-Tekhnicheskie Problemy Razrabotki Poleznykh Iskopaemykh, 2021, No. 1, pp. 118-136. https://doi.org/10.15372/FTPRPI20210112.
Rights and permissions
About this article
Cite this article
Kondrat’ev, S.A. ACTION OF PHYSISORBED COLLECTOR IN PARTICLE–BUBBLE ATTACHMENT. J Min Sci 57, 106–122 (2021). https://doi.org/10.1134/S1062739121010129
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062739121010129