Abstract
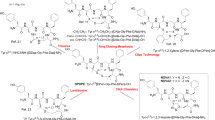
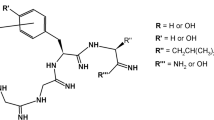
The dynorphin (Dyn) A analog arodyn (Ac[Phe1-3,Arg4,d-Ala8]Dyn A-(1–11)NH2) is a κ opioid receptor-selective antagonist, but as a linear peptide it is conformationally flexible and subject to metabolism by proteases. To restrict its conformational flexibility both short- and long-range cyclizations via ring-closing metathesis (RCM) involving residues in both the N-terminal “message” and C-terminal “address” sequences were explored. Cyclization between allyglycine (AllGly) residues in positions 5 and 8 yielded peptides with the highest κ opioid receptor affinity (Ki = 54 and 63 nM) and selectivity for κ over µ receptors (185- and 149-fold) comparable to arodyn (175-fold), with similar results for the peptides with the cis and trans double bond configurations; both isomers exhibited competitive antagonism of κ opioid receptors with potencies similar to arodyn. The minor cis isomer cyclized between AllGly residues in positions 2 and 8 exhibited similar κ receptor affinity to arodyn, but lacked selectivity for κ over µ opioid receptors. These results provide important structure-activity relationship information for cyclization of arodyn that we are using in the design of the next generation of cyclic arodyn analogs.






Similar content being viewed by others
Notes
Unless otherwise indicated, all amino acids are the L-isomer, and standard abbreviations are used according to the IUPAC-IUB Joint Commission of Biochemical Nomenclature [27]
References
Aldrich JV, McLaughlin JP. Peptide kappa opioid receptor ligands: potential for drug development. AAPS J. 2009;11:312–22. https://doi.org/10.1208/s12248-009-9105-4
Carroll FI, Carlezon WA Jr. Development of kappa opioid receptor antagonists. J Med Chem. 2013;56:2178–95. https://doi.org/10.1021/jm301783x
Urbano M, Guerrero M, Rosen H, Roberts E. Antagonists of the kappa opioid receptor. Bioorg Med Chem Lett. 2014;24:2021–32. https://doi.org/10.1016/j.bmcl.2014.03.040
Carlezon WA Jr, Krystal AD. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety. 2016;33:895–906. https://doi.org/10.1002/da.22500
Helal MA, Habib ES, Chittiboyina AG. Selective kappa opioid antagonists for treatment of addiction, are we there yet? Eur J Med Chem. 2017;141:632–47. https://doi.org/10.1016/j.ejmech.2017.10.012
Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7:E704–22. https://doi.org/10.1208/aapsj070371
Guerrero M, Urbano M, Kim EK, Gamo AM, Riley S, Abgaryan L, et al. Design and synthesis of a novel and selective kappa opioid receptor (KOR) antagonist (BTRX-335140). J Med Chem. 2019;62:1761–80. https://doi.org/10.1021/acs.jmedchem.8b01679
Bennett MA, Murray TF, Aldrich JV. Identification of arodyn, a novel acetylated dynorphin A-(1-11) analogue, as a κ opioid receptor antagonist. J Med Chem. 2002;45:5617–9. https://doi.org/10.1021/jm025575g
Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–9. https://doi.org/10.1016/j.ejphar.2007.05.007
Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci USA. 2009;106:18396–401. https://doi.org/10.1073/pnas.0910180106
Fang WJ, Murray TF, Aldrich JV. Design, synthesis, and opioid activity of arodyn analogs cyclized by ring-closing metathesis involving Tyr(allyl). Bioorg Med Chem. 2018;26:1157–61. https://doi.org/10.1016/j.bmc.2017.11.029
Gisemba SA, Ferracane MJ, Murray TF, Aldrich JV. Conformational constraint between aromatic residue side chains in the “message” sequence of the peptide arodyn using ring closing metathesis results in a potent and selective kappa opioid receptor antagonist. J Med Chem. 2021;64:3153–64. https://doi.org/10.1021/acs.jmedchem.0c01984
Fu GC, Grubbs RH. The application of catalytic ring-closing olefin metathesis to the synthesis of unsaturated oxygen heterocycles. J Am Chem Soc. 1992;114:5426–7. https://doi.org/10.1021/ja00039a065
Miller SJ, Blackwell HE, Grubbs RH. Application of ring-closing metathesis to the synthesis of rigidified amino acids and peptides. J Am Chem Soc. 1996;118:9606–14. https://doi.org/10.1021/ja961626l
Stymiest JL, Mitchell BF, Wong S, Vederas JC. Synthesis of oxytocin analogues with replacement of sulfur by carbon gives potent antagonists with increased stability. J Org Chem. 2005;70:7799–809. https://doi.org/10.1021/jo050539l
Reichwein JF, Versluis C, Liskamp RMJ. Synthesis of cyclic peptides by ring-closing metathesis. J Org Chem. 2000;65:6187–95. https://doi.org/10.1021/jo000759t
Nutt RF, Veber DF, Saperstein R. Synthesis of nonreducible bicyclic analogs of somatostatin. J Am Chem Soc. 1980;102:6539–45. https://doi.org/10.1021/ja00541a025
Vig BS, Murray TF, Aldrich JV. Synthesis of novel basic head-to-side chain cyclic dynorphin A analogs: strategies and side reactions. Biopolymers. 2003;71:620–37. https://doi.org/10.1002/bip.10591
Berezowska I, Chung NN, Lemieux C, Wilkes BC, Schiller PW. Cyclic dermorphin tetrapeptide analogues obtained via ring-closing metathesis. Acta Biochim Pol. 2006;53:73–6.
Berezowska I, Chung NN, Lemieux C, Wilkes BC, Schiller PW. Dicarba analogues of the cyclic enkephalin peptides H-Tyr-c[D-Cys-Gly-Phe-D(or L)-Cys]NH2 retain high opioid activity. J Med Chem.2007;50:1414–7. https://doi.org/10.1021/jm061294n
Mollica A, Guardiani G, Davis P, Ma SW, Porreca F, Lai J. et al.Synthesis of stable and potent δ/μ opioid peptides: analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH by ring-closing metathesis.J Med Chem. 2007;50:3138–42. https://doi.org/10.1021/jm061048b
Berezowska I, Lemieux C, Chung NN, Wilkes BC, Schiller PW. Cyclic opioid peptide agonists and antagonists obtained via ring-closing metathesis. Chem Biol Drug Des. 2009;74:329–34. https://doi.org/10.1111/j.1747-0285.2009.00867.x
Stefanucci A, Lei W, Pieretti S, Dimmito MP, Luisi G, Novellino E, et al. Novel cyclic biphalin analogues by ruthenium-catalyzed ring closing metathesis: in vivo and in vitro biological profile. ACS Med Chem Lett. 2019;10:450–6. https://doi.org/10.1021/acsmedchemlett.8b00495
Fang WJ, Cui Y, Murray TF, Aldrich JV. Design, synthesis, and pharmacological activities of dynorphin A analogues cyclized by ring-closing metathesis. J Med Chem. 2009;52:5619–25. https://doi.org/10.1021/jm900577k
Gisemba SA, Aldrich JV. Optimized ring closing metathesis reaction conditions to suppress desallyl side products in the solid-phase synthesis of cyclic peptides involving tyrosine(O-allyl). J Org Chem. 2020;85:1407–15. https://doi.org/10.1021/acs.joc.9b02345
Bennett MA, Murray TF, Aldrich JV. Structure-activity relationships of arodyn, a novel acetylated kappa opioid receptor antagonist. J Pept Res. 2005;65:322–32. https://doi.org/10.1111/j.1399-3011.2005.00216.x
Cornishbowen A. Nomenclature and symbolism for amino acids and peptides: recommendations 1983. Eur J Biochem. 1984;138:9–37. https://doi.org/10.1111/j.1432-1033.1984.tb07877.x
Fang WJ, Bennett MA, Aldrich JV. Deletion of Ac-NMePhe1 from [NMePhe1]arodyn under acidic conditions: 1. Effects of cleavage conditions and N-terminal functionality. Biopolymers. 2011;96:97–102. https://doi.org/10.1002/bip.21496
Bovey FA, Mirau PA, Gutowsky HS. Nuclear Magnetic Resonance Spectroscopy. 2nd ed. (Academic Press, San Diego, 1988). https://doi.org/10.1002/pol.1990.140280106.
Braun S, Kalinowski HO, Berger S. 150 and More Basic NMR Experiments. 2nd ed. (Wiley-VCH, Weinheim, 1998).
Silverstein RM, Webster FX. Spectrometric Identification of Organic Compounds. (John Wiley & Sons, Inc., New York, 1997).
Arttamangkul S, Ishmael JE, Murray TF, Grandy DK, DeLander GE, Kieffer BL, et al. Synthesis and opioid activity of conformationally constrained dynorphin A analogues. 2. Conformational constraint in the “address” sequence. J Med Chem. 1997;40:1211–8. https://doi.org/10.1021/jm960753p
Fang WJ, Bennett MA, Murray TF, Aldrich JV. Deletion of Ac-NMePhe1 from [NMePhe1]arodyn under acidic conditions: 2. Effects of substitutions on pharmacological activity. Biopolymers. 2011;96:103–10. https://doi.org/10.1002/bip.21495
Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–8. https://doi.org/10.1016/0003-2697(70)90146-6
Solé NA, Barany G. Optimization of solid-phase synthesis of [Ala8]-dynorphin A. J Org Chem. 1992;57:5399–403. https://doi.org/10.1021/jo00046a022
Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. https://doi.org/10.1016/0006-2952(73)90196-2
Ross NC, Reilley KJ, Murray TF, Aldrich JV, McLaughlin JP. Novel opioid cyclic tetrapeptides: Trp isomers of CJ-15,208 exhibit distinct opioid receptor agonism and short-acting kappa opioid receptor antagonism. Br J Pharmacol. 2012;165:1097–108. https://doi.org/10.1111/j.1476-5381.2011.01544.x
Schild HO. pA, a new scale for the measurement of drug antagonism. Br J Pharmacol Chemother. 1947;2:189–206. https://doi.org/10.1111/j.1476-5381.1947.tb00336.x
Acknowledgements
This research was supported by the National Institute on Drug Abuse grant R01 DA018832. We thank Ms. Bridget Sefranek for her excellent technical assistance with the radioligand and [35S]GTPγS binding assays.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is dedicated to Dr. Gary Gunewald, an outstanding chemist who has contributed so much to the field of medicinal chemistry and a wonderful colleague.
Supplementary information
Rights and permissions
About this article
Cite this article
Fang, WJ., Murray, T.F. & Aldrich, J.V. Analogs of the κ opioid receptor antagonist arodyn cyclized by ring-closing metathesis retain κ opioid receptor affinity, selectivity and κ opioid receptor antagonism. Med Chem Res 30, 1397–1407 (2021). https://doi.org/10.1007/s00044-021-02758-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02758-x