Abstract
Leaching titaniferous magnetite concentrate with alkali solution of high concentration under high temperature and high pressure was utilized to improve the grade of iron in iron concentrate and the grade of TiO2 in titanium tailings. The titaniferous magnetite concentrate in use contained 12.67% TiO2 and 54.01% Fe. The thermodynamics of the possible reactions and the kinetics of leaching process were analyzed. It was found that decomposing FeTiO3 with NaOH aqueous solution could be carried out spontaneously and the reaction rate was mainly controlled by internal diffusion. The effects of water usage, alkali concentration, reaction time, and temperature on the leaching procedure were inspected, and the products were characterized by X-ray diffraction, scanning electron microscope, and energy dispersive spectroscopy, respectively. After NaOH leaching and magnetic separation, the concentrate, with Fe purity of 65.98% and Fe recovery of 82.46%, and the tailings, with TiO2 purity of 32.09% and TiO2 recovery of 80.79%, were obtained, respectively.
Similar content being viewed by others
1 Introduction
Titaniferous magnetite ore (TMO) can be utilized for production of pig iron as main product and titanium compound as byproduct [1]. The ore is a kind of symbiotic magnetite, mainly in the form of magnetite, and contains a small amount of ilmenite and titanomagnetite [2, 3]. TMO is hard to be dissociated into monomer. In the existing iron concentrating process, TMO goes into iron concentrate, and ilmenite goes into concentrate and tailings. The concentrated TMO contains more than 10% TiO2, where the available iron grade is not high, and moreover, titanium enters into blast furnace slag during the iron smelting procedure and cannot be economically recovered [4]. Due to the inherent dense structure and the symbiotic relationship between iron and titanium in TMO, only by making titanium and iron dissociate on the lattice level, can we get high quality iron concentrate and titanium tailings. Therefore, TMO as a typical poly metallic paragenic source of iron is difficult to treat [5]. The separation of titanium and iron from ilmenite is completed by chemical process. Pre-reduction is the thermo-chemical beneficiation process which is very useful technique for upgradation of metal values from complex low grade ore. The previous research has been based on lump TMO by gas–solid and solid–solid reduction, but the degree of reducibility is not very high and most of the ilmenite grains are irreducible [6]. The carbon- or hydrogen-based reduction is used in common [7,8,9,10,11,12]. Pre-treatment by multiple heating to high temperature (1373 K and 1473 K, respectively) and subsequently sudden cooling to room temperature by water successfully increase the porosity as well as many fissures in dense grain, which significantly enhance the degree of reduction of TMO [13]. Studies have been reported by various researchers on the effects of pre-oxidation, the addition of alkali metals such as sodium sulfate, sodium carbonate, borax, and ferrosilicon for enhancing the reducibility of TMO [14]. B.C. JENA et al. [15] used a combined hot-wet process to recover iron and titanium from the vanadium-titanium magnetite deposit. Selective reduction smelting of the ore can achieve a titanium recovery rate of more than 90%. Zhao et al. [16] proposed a method for extracting iron, vanadium, and titanium from titanium magnetite concentrates, mainly through concentrate reduction, magnetic separation, and hydrochloric acid treatment of titanium-containing tailings slag. This new process can realize the recovery of iron and titanium; the rates were 88.3% and 93.7%, respectively. Chen et al. [17] used molten salt roasting and water leaching processes to treat titanium magnetite concentrate, and the recovery rate of titanium in the titanium-rich slag reached 96.3% under the optimal process conditions.
Due to high temperature and energy consumption for ilmenite reduction, most processes mentioned above still have not yet been employed industrially. Therefore, it is of great significance to study the separation of titanium and iron in titaniferous magnetite concentrate. In this paper, high-pressure alkaline leaching method was used to obtain high-quality iron concentrate and titanium tailings from TMO by means of the impregnating and etching effects of high concentration NaOH aqueous solution.
2 Experimental
2.1 Raw Material
Titanium magnetite concentrate is a mineral obtained after iron beneficiation of titanium magnetite from the Midi Concentrator in Panzhihua, China. The titanium magnetite concentrate particle size is fine, and the content of minerals with a particle size of less than 0.074 mm reaches about 62%. Its compositions were expressed as the forms of Fe2O3, FeO, TiO2, etc., and listed in Table 1. The major contents were 12.67% TiO2 and 54.01% Fe. The phase analysis results of the vanadium–titanium magnetite concentrate are shown in Table 2 and 3.
2.2 Experimental Procedure
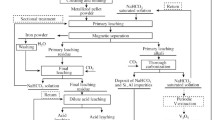
In order to ensure the representativeness of the test sample, this experiment adopts the method of sample splitting. Weigh 5 kg of mineral powder and reduce the mass to 150 g after sample splitting, the leaching reaction of 150 g titaniferous magnetite concentrate with a certain amount of NaOH aqueous solution was carried out in a sealed autoclave (1 L capacity, Parr, the USA). The operational process was to fill the autoclave with the materials, tighten the cover, purge the reactor with N2, and then stir the mixture at 600 r/min; meanwhile, heat the autoclave, after keeping the mixture react at a temperature between 300 and 400 °C for a certain time, cool the mixture to room temperature, and filter, wash, and dry the powder. Magnetic separation tube (XCGS-Φ 50, China) with the largest magnetic induction intensity of 250 KA/M was used to separate the powder to obtain iron concentrate and titanium tailings at an electric current of 1.25 A. Figure 1 depicts the process flow. The high-pressure reactor of instrument parameters and the main technical parameters of magnetic tube are shown in Tables 4 and 5, respectively.
2.3 Analysis
The determination of Fe (total iron) content and TiO2 content in the samples were performed by using the potassium dichromate volumetric method and the ammonium ferric sulfate volumetric method, respectively. The characterization of phase composition and morphology of the solid samples were performed on an X-ray diffractometer (XRD: X’Pert Powder, PANalytical B.V., Netherlands), scanning electron microscopy (SEM: ZEISS, IGMAHD), and energy dispersive spectroscopy (EDS: Oxford, X-Max).
3 Results and Discussion
3.1 Thermodynamic Analysis
In Fig. 2 of characterization of the raw mineral in use, XRD spectrum revealed that the mineral mainly consisted of ilmenite in space group of rhombohedral, of which the main diffraction peak and sub-strong diffraction peak are 32.59° and 35.23°; SEM picture showed the dense appearance of the raw mineral, EDS of the designed area exhibited Ti, and Fe distribution in uniform and EDS of the marking point provided the weight percentage of 34.13% Fe and 31.28% Ti. The mineral feature determined chemical separation rather than physical separation. Therefore, we used the chemical reaction of ilmenite with NaOH aqueous solution to leach Ti element and separate Ti and Fe.
The three possible leach reactions were as follows.
The Gibbs free energy change ΔGof a reaction is obtained by Eq. (1).
where ΔGΘ is the standard Gibbs free energy change, R the ideal gas constant, 8.314J ⋅ K−1 ⋅ mol−1, T the reaction temperature, \( {P}_{H_2O} \) is the vapor pressure of water, and pΘ = 1atm.
The leaching reaction was carried out in a closed high-temperature and high-pressure reactor, with water usage of 210–480 g. The curve of actual pressure changing with temperature (P-T curve) was shown in Fig. 3, where the pressure range was 1–180 atm. According to the three-phase balance diagram of water [18], water existed in a gaseous form under the above conditions. Therefore, the influence of the vapor pressure of water \( {P}_{H_2O} \) on reaction thermodynamics must be considered. The reaction system pressure was mainly controlled by the amount of water.
According to the heat capacity, standard enthalpy and entropy of each material involved in the reaction equation; ΔGΘwas calculated. Combining the experimental measured P-T curves under different water usages, ΔG-T curves of each leaching reaction were computed, as shown in Fig. 3.
Figure 3 showed that under the conditions of the temperature of 100–400 °C and the water usage of 210–480 g, the ΔG values of the three reactions were less than zero, indicating that leaching titanium from ilmenite with NaOH aqueous solution was a spontaneous process. With the increase of the temperature, ΔG decreased, so the higher temperature was advantageous to the titanium leaching and the more favorable for the reactions (3). When the temperature was higher than about 300 °C, the ΔG values of reactions (1) and (2) increased. Of the three reactions, reaction (2) was more likely to occur under the same temperature. ΔG of each reaction was also impacted by water usage. With the increase of the water usage, ΔG increased, and when the water usage was higher than about 410 g, the ΔG change got smaller.
Due to the complex composition of the ore, the dissolution was complicated under the conditions of high temperature, pressure, and concentrated alkali. These three products might not be the final products. As a preliminary reaction, the leaching process was completely thermodynamically feasible.
3.2 Kinetic Analysis
Titanium leaching occurred when NaOH reacted with titaniferous magnetite concentrate, so it was considered that the reaction process was applicable to the shrinkage core model [19]. According to the linear correlation coefficients of Eq. (2) and Eq. (3), the kinetic control steps of the reaction process were judged.
where t is the reaction time, k the rate constant, and X the extent of reaction (i.e., 0 = no reaction, 1 = complete digestion). Equation (2) represents that chemical reactions at the surface is the main determinant of reaction rate; Equation (3) represents that diffusion through a reacted layer of increasing thickness is the main determinant of reaction rate.
Base on the recovery (X) of TiO2 in tailings obtained experimentally under three different leaching conditions, the relationships between \( 1+2\left(1-X\right)-3{\left(1-X\right)}^{\raisebox{1ex}{$2$}\!\left/ \!\raisebox{-1ex}{$3$}\right.} \) and reaction time and between \( 1-{\left(1-X\right)}^{\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$3$}\right.} \) and reaction time were obtained (see Fig. 4). Comparison with the two relationships under the same leaching condition indicated a better linear relation between \( 1+2\left(1-X\right)-3{\left(1-X\right)}^{\raisebox{1ex}{$2$}\!\left/ \!\raisebox{-1ex}{$3$}\right.} \) and reaction time. Therefore, product diffusion was considered the control factor of reaction rate. Furthermore, it was found that the linear correlation coefficient in Eq. (3), obtained in case (c) under higher reaction temperature, was doubled as compared with cases (a) and (b).
Relationships between \( 1-{\left(1-X\right)}^{\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$3$}\right.} \) and time and between \( 1+2\left(1-X\right)-3{\left(1-X\right)}^{\raisebox{1ex}{$2$}\!\left/ \!\raisebox{-1ex}{$3$}\right.} \) and time (In the cases of (a) temperature 300 °C, NaOH concentration 40%, and water usage 410 g; (b) temperature 310 °C, NaOH concentration 45%, and water usage 315 g; (c) temperature 320 °C, NaOH concentration 40%, and water usage 410 g)
3.3 Product Characterization
3.3.1 Concentrates
SEM images of concentrates C1–C9 in Fig. 5 showed that there were signs of etching effect on concentrate C1 obtained by 150 g ore reacting with 40% NaOH in 410 g water at 300 °C only after 15 min; after 30 min, the parallel grooves on concentrate C2 were produced; for concentrate C3 obtained after 60 min, the surface of the large grain was etched into grid grooves, while the edges and corners of the small particles were no longer clear; after 120 min, the gridded ravines of concentrate C4 were deepened, the small cubic particles of 300–650 nm appeared on the observed big grain, and some of them fell off; after 180 min, there still existed the particles hard to be etched in concentrate C5; when reacting at 320 °C for 1 h, the etching effect on concentrate C6 was heavier than that of C3; with the increase of temperature to 360 °C, concentrate C7 was etched into finer cubic particles, about 250–500 nm in size; for concentrate C8 obtained by etching 150 g ore with 293 g NaOH and 210 g water at 340 °C for 1 h, the surface etching was much heavier that the size of the etched particles became much smaller, 200–400 nm, because of the high NaOH concentration of 58.25% w/w, but the deeper leaching did not take place due to high viscosity of the reaction medium; with the increase of the water usage to 315 g, the etching effect on or under surface was much heavier that the edges of the formed cube particles became much rounded and more gaps and voids appeared on concentrate C9.
SEM images of concentrates C1–C9 (concentrates C1–C5 obtained under the conditions of 150 g ore, 300 °C, 40% NaOH, 410 g water, and different reaction times of 15 min, 30 min, 60 min, 90 min, and 120 min, respectively; concentrates C6-C7 obtained under the conditions of 150 g ore, 40% NaOH, 410 g water, 60 min, and different reaction temperatures of 320 °C and 360 °C, respectively; concentrates C8-C9 obtained under the conditions of 150 g ore, 293 g NaOH, 300 °C for 1 h, and different water usage of 210 g and 315 g, respectively)
The data in Table 4 of EDS measurements of marking points on SEM photographs of concentrates C1–C9 in Fig. 5 indicated that the leaching reaction was rapid and titanium on the surface of C1 was significantly reduced at 15 min; with the extension of time, the ratio of iron to titanium on the surface of some mineral particles, such as C2–1 or C2–2, C3–1 or C3–2, and C5, did not change any more, and on the surface of other mineral particles, such as C4–1 or C4–2 increased further (C2–1 and c2–2 were the marking points observed on the surface of a same grain of sample C2; C3–1 and c3–2 the points of a bigger grain and a smaller particle of sample C3, respectively; C4–1 and c4–2 the points of a eroded grain and a broken grain of sample C4, respectively); with the increase of temperature, the ratio of iron to titanium on the surface of C3–2, C6, and C7 increased; with the increase of water usage, the ratio of iron to titanium on the surface of C8 and C9 increased, either (Table 6).
XRD spectra of concentrates C1–C9 in Fig. 6 displayed that the main diffraction peak of ilmenite almost disappeared in concentrate C1 and did not exist in concentrates C2-C9, proving that the leaching reaction rate was fast at low temperature; the appeared diffraction peaks belonged to Fe3O4 in the space group of Cubic Fd-3 m, of which the main diffraction peak and sub-strong diffraction peak are 35.44° and 62.55°, respectively. FeO, as the product of the leach reaction, was easy to be oxidized by O2 dissolved in NaOH aqueous solution during washing and filtering processes and changed into Fe3O4.
3.3.2 Tailings
Corresponding to C1–C9 concentrates, there were tailings T1–T9, respectively. SEM images of tailings T1–T9 in Fig. 7 showed that under conditions of 150 g ore, 40% NaOH, 410 g water, and reacting at 300 °C, when the reaction times were 15 min, 30 min, 60 min, 120 min and 180 min, tailings T1–T5 in diameters of about 165 nm, 110 nm, 94 nm, 65 nm, and 63 nm, and in lengths of about 883 nm, 1200 nm, 3294 nm, 4025 nm, and 6382 nm were obtained, respectively, showing with the increase of reaction time, acicular substances in tailings gradually formed, and their length-diameter ratio increased; when the reaction temperatures were 320 and 360 °C, tailings T6 and T7 in diameters of about 64 nm and 159 nm, and in lengths of about 3273 nm and 1817 nm were obtained, showing with the increase of temperatures, the length-diameter ratio of acicular substances decreased, forming rod material; when the water usages were 210 g, there were no acicular or rod substances in tailings T8, and when the water usages were 315 g, there appeared rod substances in tailings T9, showing water providing environment for a crystal growing.
The data in Table 5 of EDS measurements of marking points on SEM photographs of tailings T1–T9 in Fig. 7 indicated that ilmenite erosion was a very complicated process. Titanium leaching of different particles, such as T1–1, T1–2, and T1–3, was inconsistent; except for titanium leaching, there was the leaching of other components, e.g., T1–2 contained 8.19% Si. The data of EDS measurements of tailings T1–T9 gave the regular conclusions that with the extension of reaction time, the increase of temperature, and water usage, the ratio of titanium to iron of T1–T9 increased (Table 7).
XRD spectra of tailings T1–T9 in Fig. 8 displayed that in tailings T1–T9, the characteristic diffraction peaks of ilmenite almost completely disappeared also, but the characteristic diffraction peaks of Fe3O4 existed strongly; this was because the magnetic tube was difficult to separate the Fe3O4 particles less than 10 μm from tailings. In XRD spectra, the characteristic diffraction peak of Na2(TiO)(SiO4) in space group of tetragonal P4/nmm was found at 32.70° in tailings T1–T7, the characteristic diffraction peak of TiO2 rutile in space group of tetragonal P42/mnm was found at 27.46° in tailings T3–T6 and the characteristic diffraction peak of Na4Ti5O12 in space group of monoclinic C2/m was found at 14.07° in tailing T7, respectively. These products were formed by the following reasons: under the caustic condition, SiO2 became Na4SiO4, and the Ti in FeTiO3 became TiO2+, Na2TiO3, Na2Ti2O5, and Na2Ti3O7. TiO2+ substituted the Na+ in Na4SiO4 and formed Na2(TiO)(SiO4); the hydrolysis of Na2TiO3, Na2Ti2O5, and Na2Ti3O7 formed TiO2; the aggregation of Na2Ti2O5 and Na2Ti3O7 formed Na4Ti5O12.
This is a very complex hydrothermal reaction under high temperature, high pressure, and high alkalinity, so that the titanium-containing materials could be grown into crystals with certain shapes directionally.
3.4 Effects of Reaction Conditions on Separation
The effects of water usage, NaOH concentration, reaction temperature, and reaction time on the separation were investigated by single factor experiments.
Although after 15 min the leaching reaction could take place at 300 °C, the mass transfer and diffusion rate of leached Ti in solid product FeO were slow. Therefore, at 60 min, the purity and recovery of TiO2 of tailings (18.49% and 38.24%, respectively) were low, and so were the purity and recovery of Fe of concentrates (59.28% and 80.12%, respectively). The separation effect can only be achieved by extending the reaction time, as shown in Fig. 9a.
Influences of reaction conditions on separation indices ((a) influence of reaction time, under the other conditions of 150 g ore, 300 °C, 40% NaOH, and 410 g water; (b) influence of reaction temperature, under the other conditions of 150 g ore, 40% NaOH, 410 g water, and 60 min; (c) influence of NaOH concentration, under the other conditions of 150 g ore, 410 g water, and 340 °C for 1 h; (d) influence of water usage, under the other conditions of 150 g ore, 293 g NaOH, and 300 °C for 1 h. ■-Fe purity of concentrate, ●-Fe recovery of concentrate, ▲-TiO2 purity of tailings, ▼-TiO2 recovery of tailings)
The effect of temperature on the separation was significant, as shown in Fig. 9b. By increasing the temperature, ΔG of the reaction was decreased and the random thermal velocity of all particles was increased. When the leaching reaction took place at 360 °C for 60 min, the purity and recovery of TiO2 of tailings increased simultaneously, which were 31.48% and 74.04%, respectively; meanwhile, the purity and recovery of Fe of concentrates increased to 64.49% and 83.34%, respectively. Therefore, increasing the temperature can improve the separation ability and efficiency.
The leaching reaction should be carried out in a NaOH solution of high concentration, higher than weight percent of 30% (see Fig. 9c). Except for as a reactant, the alkali was used to improve the polarity of the medium. However, NaOH concentration should not be too high; otherwise, the solution viscosity will increase, which will not be conducive to the deep penetration of NaOH into the mineral particles. Here, the appropriate concentration of NaOH was 40%.
Figure 9d showed that an appropriate increase in water usage was beneficial to the separation between Fe and Ti in titanomagnetite. Although a higher water content increased the pressure of reactor leading to the increase of ΔG of the leaching reaction, it helped NaOH immersing, Ti leaching, and directionally crystallizing in dynamics.
According to the above experimental results, a good separation condition was that water usage was 410 g, NaOH concentration 40%, temperature 400 °C, and reaction time 1 h. Under this condition, the concentrate with purity values of 65.98% Fe and 3.65% TiO2 and the tailings with purity values of 28.78% Fe and 32.09% TiO2 were obtained.
The later work proved that this needle or bar titanium containing mixture (tailings) is a kind of additive for welding wire, which can improve the tensile strength and yield strength of welding seam.
4 Conclusion
The new process of “chemical-physical” combined mineral processing is used to treat titaniferous magnetite concentrate, which realizes the efficient separation of titanium and iron in the titaniferous magnetite concentrate. After separation, the iron grade of the concentrate is increased from 54 to more than 65%, and TiO2 in the titanium tailings has been raised from 12.67 to over 32%. It was thermodynamic feasible to leach titanium from titaniferous magnetite ore by NaOH aqueous solution under high temperature and high pressure. In the leaching process, the interfacial reaction rate was faster than the internal diffusion rate. After NaOH leaching and magnetic separation, in the concentrate, there were small Fe3O4 particles in shape of cube, and in the tailings, there were needle-like or rod-like mixtures containing titanium. At low temperature, an acicular Ti-containing mixture could form in a long time reaction, while at high temperature, a rod Ti-containing mixture could form in a short time reaction. In this leaching experiment, the pressure of the reaction system was controlled by water usage. When water usage was large, it was beneficial for NaOH to immerse deeply into the mineral particles, for Ti-ion to be leached from the mineral particles and for Ti-ion to crystallize directionally. This process solves the problem of low iron grade in the smelting furnace, realizes the efficient recovery of TiO2, opens a new way for the rational and efficient application of titanium resources in China, and can realize the optimal allocation of mineral resources and promote economic growth.
References
Wang YM, Yuan ZF, Guo ZC, Tan QQ, Li ZY, Jiang WZ (2008) Reduction mechanism of natural ilmenite with graphite. Trans Nonferrous Metals Soc China 18:962–968. https://doi.org/10.1016/S1003-6326(08)60166-1
Liu S, Guo Y, Qiu G, Jiang T, Chen F (2014) Solid-state reduction kinetics and mechanism of pre-oxidized vanadium–titanium magnetite concentrate. Trans Nonferrous Metals Soc China 24:3372–3377. https://doi.org/10.1016/s1003-6326(14)63479-8
Tan P, Hu HP, Zhang L (2011) Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration. Trans Nonferrous Metals Soc China 21:1414–1421. https://doi.org/10.1016/s1003-6326(11)60875-3
Wang YM, Wang XW, He YH, Lou TP, Sui ZT (2008) Isothermal precipitation and growth process of perovskite phase in oxidized titanium bearing slag. Trans Nonferrous Metals Soc China 18:459–462. https://doi.org/10.1016/S1003-6326(08)60081-3
Si XG, Lu XG, Li CW, Li CH, Din WZ (2012) Phase transformation and reduction kinetics during the hydrogen reduction of ilmenite concentrate. Int J Miner Metall Mater 19:384–390. https://doi.org/10.1007/s12613-012-0568-4
Saikat S, Manik CG, Tapan KB, Siddhartha M, Rajib D (2013) Mineralogy and carbothermal reduction behaviour of vanadium-bearing titaniferous magnetite ore in Eastern India. Int J Miner Metall Mater 20:917–924. https://doi.org/10.1007/s12613-013-0815-3
Eungyeul P, Oleg O (2004) Reduction of titania-ferrous ore by hydrogen. ISIJ Int 44:990–1005. https://doi.org/10.2355/isijinternational.44.999
Kucukkaragoz CS, Eric RH (2006) Solid state reduction of a natural ilmenite. Miner Eng 19:334–337. https://doi.org/10.1016/j.mineng.2005.09.015
Wang Y, Yuan Z, Matsuura H, Tsukihashi F (2009) Reduction extraction kinetics of titania and iron from an ilmenite by H2-Ar gas mixtures. ISIJ Int 49:164–170. https://doi.org/10.2355/isijinternational.49.164
Krasikov SA, Maiorov LA, Ponomarenko AA, Zhidovinova SV, Savvinova AA (2011) Separation of elements in processing titanomagnetite concentrates. Steel Transl 41:752–755. https://doi.org/10.3103/S0967091211090105
Hu T, Lv XW, Bai CG, Lun ZG, Qiu GB (2013) Reduction behavior of Panzhihua titanomagnetite concentrates with coal. Metall Mater Trans B Process Metall Mater Process Sci 44:252–260. https://doi.org/10.1007/s11663-012-9783-7
Hu T, Sun T, Kou J, Geng C, Wang X, Chen C (2017) Recovering titanium and iron by co-reduction roasting of seaside titanomagnetite and blast furnace dust. Int J Miner Process 165:28–33. https://doi.org/10.1016/j.minpro.2017.06.003
Samanta S, Mukherjee S, Dey R (2015) Upgrading metals via direct reduction from poly-metallic titaniferous magnetite ore. J Miner Met Mater Soc 67:467–476. https://doi.org/10.1007/s11837-014-1203-9
Chen DS, Song B, Wang LN, Qi T, Wang Y, Wang WJ (2011) Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner Eng 24:864–869. https://doi.org/10.1016/j.mineng.2011.03.018
Jena B, Dresler W, Reilly I (1995) Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, Manitoba, Canada. Miner Eng 8:159–168
Zhao LS, Wang LN, Qi T, Chen DS, Zhao HX, Liu YH (2014) A novel method to extract iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 149:106–109. https://doi.org/10.1016/j.hydromet.2014.07.014
Chen DS, Zhao LS, Liu YH, Qi T, Wang JC, Wang LN (2013) A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J Hazard Mater 244-245:588–595. https://doi.org/10.1016/j.jhazmat.2012.10.052
Silberberg MS (2000) Chemistry: the molecular nature of matter and change (second edition) [M]. The McGraw-Hill Companies, Inc
Haverkamp RG, Kruger D, Rajashekar R (2016) The digestion of New Zealand ilmenite by hydrochloric acid. Hydrometallurgy 163:198–203. https://doi.org/10.1016/j.hydromet.2016.04.015
Acknowledgements
Thanks to the people helping with this work, and acknowledges the valuable suggestions and reviews from the peer reviewers.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, K., Wang, S., Song, R. et al. Leaching Titaniferous Magnetite Concentrate by Alkaline Aqueous Solution. Mining, Metallurgy & Exploration 38, 1721–1730 (2021). https://doi.org/10.1007/s42461-021-00387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00387-x