Abstract
Purpose
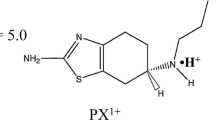
Pulsed direct current (PDC) iontophoresis, by allowing skin depolarization, was suggested to provide more efficient ion transport, but the extent of its enhancement effect was unclear. PDC could also offer electric-customized drug delivery. This study examined the effect of PDC iontophoresis on transdermal delivery of pramipexole dihydrochloride (PXCl).
Methods
Iontophoretic delivery of PXCl across human epidermal membrane from pH 7.0 solution was conducted in vitro using continuous direct current (DC) and 6- and 12-cycle PDC iontophoresis (0.5 mA/cm2 and total applied duration of 6 h). Different parameters of PDC iontophoresis were studied, including current density (0.1, 0.2 and 0.5 mA/cm2) and on-off current dosing pattern (1 h/3 h, 0.5 h/3.5 h, and 0.2 h/3.8 h).
Results
Both 6- and 12-cycle PDC iontophoresis protocols provided modulation of the permeation profile but delivered smaller amounts of PXCl (396 and 400 μg/cm2, respectively) as compared with continuous DC iontophoresis (482 μg/cm2) at 24 h after 0.5 mA/cm2 and 180 mA/cm2 × min current dose application. Increasing applied current density from 0.1 to 0.5 mA/cm2 increased the PDC iontophoretic flux of PXCl linearly from 5.3 to 14.6 μg/cm2·h (R2 = 0.887). Varying the current level and duration but at the same applied current dose (36 mA/cm2 × min), the total amount of PXCl delivered by PDC iontophoresis at 24 h was independent of the on-off dosing pattern studied (114–128 μg/cm2).
Conclusions
The results indicate that PDC iontophoresis can benefit transdermal delivery of PXCl in terms of controlling its permeation but does not enhance iontophoretic transport compared to continuous DC iontophoresis under the conditions studied.






Similar content being viewed by others
Abbreviations
- DC:
-
Direct current
- HCl:
-
Hydrochloric acid
- HEM:
-
Human epidermal membrane
- HPLC:
-
High performance liquid chromatography
- J :
-
Flux
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- NaCl:
-
Sodium chloride
- NaOH:
-
Sodium hydroxide
- PBS:
-
Phosphate-buffered saline
- PDC:
-
Pulsed direct current
- PX:
-
Pramipexole
- PXCl:
-
Pramipexole dihydrochloride
- SD:
-
Standard deviation
- t i :
-
Transference number
References
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, et al. Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European section. Part I: early (uncomplicated) Parkinson's disease. Eur J Neurol. 2006;13(11):1170–85.
Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72(21 Suppl 4):S1–136.
Engber TM, Susel Z, Juncos JL, Chase TN. Continuous and intermittent levodopa differentially affect rotation induced by D-1 and D-2 dopamine agonists. Eur J Pharmacol. 1989;168(3):291–8.
Mouradian MM, Heuser IJ, Baronti F, Chase TN. Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson's disease. Ann Neurol. 1990;27(1):18–23.
Antonini A, Barone P, Ceravolo R, Fabbrini G, Tinazzi M, Abbruzzese G. Role of pramipexole in the management of Parkinson’s disease. CNS Drugs. 2010;24(10):829–41.
Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13(3):175–87.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3(9):318–26.
Steiger M. Constant dopaminergic stimulation by transdermal delivery of dopaminergic drugs: a new treatment paradigm in Parkinson's disease. Eur J Neurol. 2008;15(1):6–15.
Mirapex Product Monograph [Internet]. Canada: Boehringer Ingelheim (Canada) Ltd.; 2020 [updated 2020 March 19; cited 2020 July 20]. Available from: https://www.boehringer-ingelheim.ca/sites/ca/files/documents/mirapexpmen.pdf.
Saepang K, Li SK, Chantasart D. Effect of pH on iontophoretic transport of pramipexole dihydrochloride across human epidermal membrane. Pharm Res. 2021;38(4):657–68.
Kalaria DR, Patel P, Merino V, Patravale VB, Kalia YN. Controlled iontophoretic delivery of pramipexole: electrotransport kinetics in vitro and in vivo. Eur J Pharm Biopharm. 2014;88(1):56–63.
Kalaria DR, Singhal M, Patravale V, Merino V, Kalia YN. Simultaneous controlled iontophoretic delivery of pramipexole and rasagiline in vitro and in vivo: transdermal polypharmacy to treat Parkinson’s disease. Eur J Pharm Biopharm. 2018;127:204–12.
Lelawongs P, Liu JC, Chien YW. Transdermal iontophoretic delivery of arginine-vasopressin (II): evaluation of electrical and operational factors. Int J Pharm. 1990;61(3):179–88.
Patel N, Jain S, Madan P, Lin S. Influence of electronic and formulation variables on transdermal iontophoresis of tacrine hydrochloride. Pharm Dev Technol. 2015;20(4):442–57.
Nayak AK, Dey S, Pal K, Banerjee I. Iontophoretic drug delivery systems. In: Pal K, Kraatz HB, Khasnobish A, Bag S, Banerjee I, Kuruganti U, editors. Bioelectronics and Medical Devices: Woodhead Publishing. 2019; p. 393–420.
Bagniefski T, Burnette RR. A comparison of pulsed and continuous current iontophoresis. J Control Release. 1990;11(1):113–22.
Prasad R, Koul V, Anand S, Khar RK. Effect of DC/mDC iontophoresis and terpenes on transdermal permeation of methotrexate: in vitro study. Int J Pharm. 2007;333(1):70–8.
Malinovskaja K, Laaksonen T, Hirvonen J. Controlled transdermal delivery of leuprorelin by pulsed iontophoresis and ion-exchange fiber. Eur J Pharm Biopharm. 2014;88(3):594–601.
Manjunatha RG, Prasad R, Sharma S, Narayan RP, Koul V. Iontophoretic delivery of lidocaine hydrochloride through ex-vivo human skin. J Dermatolog Treat. 2020;31(2):191–9.
Hirvonen J, Hueber F, Guy RH. Current profile regulates iontophoretic delivery of amino acids across the skin. J Control Release. 1995;37(3):239–49.
Chen LLH, Chien YW. Transdermal iontophoretic permeation of luteinizing hormone releasing hormone: characterization of electric parameters. J Control Release. 1996;40(3):187–98.
Tiwari SB, Udupa N. Investigation into the potential of iontophoresis facilitated delivery of ketorolac. Int J Pharm. 2003;260(1):93–103.
Knoblauch P, Moll F. In vitro pulsatile and continuous transdermal delivery of buserelin by iontophoresis. J Control Release. 1993;26(3):203–12.
Balicki R, Sypniewski M, Ciesielska A, Szelejewski W, Zagrodzka J, Cieplucha G, inventors; Instytut Farmaceutyczny, assignee. Process for the preparation of pramipexole base and/or its salts. United States patent US 20090105483. 2009.
Chantasart D, Sa-Nguandeekul P, Prakongpan S, Li SK, Higuchi WI. Comparison of the effects of chemical permeation enhancers on the lipoidal pathways of human epidermal membrane and hairless mouse skin and the mechanism of enhancer action. J Pharm Sci. 2007;96(9):2310–26.
Raykar PV, Fung MC, Anderson BD. The role of protein and lipid domains in the uptake of solutes by human stratum corneum. Pharm Res. 1988;5(3):140–50.
Peck KD, Ghanem AH, Higuchi WI, Srinivasan V. Improved stability of the human epidermal membrane during successive permeability experiments. Int J Pharm. 1993;98(1):141–7.
Kasting GB, Bowman LA. DC electrical properties of frozen, excised human skin. Pharm Res. 1990;7(2):134–43.
Malenović A, Janić-Stojanović B, Vemić A, Ivanović D, Medenica M. Validation of a column liquid chromatographic method for the analysis of pramipexole and its five impurities. J AOAC Int. 2010;93(4):1102–12.
Li SK, Hao J, Liddell MR. Electrotransport across membranes in biological media: Electrokinetic theories and applications in drug delivery. In: Becker S, Kuznetsov A, editors. Transport in Biological Media. Philadelphia: Elsevier; 2013. p. 417–54.
Nugroho AK, Li L, Dijkstra D, Wikström H, Danhof M, Bouwstra JA. Transdermal iontophoresis of the dopamine agonist 5-OH-DPAT in human skin in vitro. J Control Release. 2005;103(2):393–403.
Cázares-Delgadillo J, Ganem-Rondero A, Quintanar-Guerrero D, López-Castellano AC, Merino V, Kalia YN. Using transdermal iontophoresis to increase granisetron delivery across skin in vitro and in vivo: effect of experimental conditions and a comparison with other enhancement strategies. Eur J Pharm Sci. 2010;39(5):387–93.
Nugroho AK, Li GL, Danhof M, Bouwstra JA. Transdermal iontophoresis of rotigotine across human stratum corneum in vitro: influence of pH and NaCl concentration. Pharm Res. 2004;21(5):844–50.
Marro D, Kalia YN, Delgado-Charro MB, Guy RH. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm Res. 2001;18(12):1701–8.
Kolli CS, Chadha G, Xiao J, Parsons DL, Babu RJ. Transdermal iontophoretic delivery of selegiline hydrochloride, in vitro. J Drug Target. 2010;18(9):657–64.
Tojo K. Mathematical model of iontophoretic transdermal drug delivery. J Chem Eng Jpn. 1989;22(5):512–8.
Wang Y, Allen LV, Li LC, Tu YH. Iontophoresis of hydrocortisone across hairless mouse skin: investigation of skin alteration. J Pharm Sci. 1993;82(11):1140–4.
Okabe K, Yamaguchi H, Kawai Y. New iontophoretic transdermal administration of the beta-blocker metoprolol. J Control Release. 1986;4(2):79–85.
Phipps JB, Gyory JR. Transdermal ion migration. Adv Drug Deliv Rev. 1992;9(2–3):137–76.
Calatayud-Pascual MA, Balaguer-Fernández C, Serna-Jiménez CE, Del Rio-Sancho S, Femenía-Font A, Merino V, et al. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharm. 2011;416(1):189–94.
Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–58.
Saliba SA, Teeter-Heyl CL, McKeon P, Ingeroll CD, Saliba EN. Effect of duration and amplitude of direct current when lidocaine is delivered by iontophoresis. Pharmaceutics. 2011;3(4):923–31.
Green PG. Iontophoretic delivery of peptide drugs. J Control Release. 1996;41(1):33–48.
Neupane R, Boddu SHS, Renukuntla J, Babu RJ, Tiwari AK. Alternatives to biological skin in permeation studies: current trends and possibilities. Pharmaceutics. 2020;12(2):152.
Guidance for Industry: Nonprescription Sunscreen Drug Products—Safety and Effectiveness Data. [Internet]. US Food and Drug Administration (FDA); 2016 [cited 2021 March 21]. Available from: https://www.fda.gov/media/94513/download.
Guidance for Industry: Transdermal and Topical Delivery Systems – Product Development and Quality Considerations [Internet]. US Food and Drug Administration (FDA); 2019 [cited 2021 March 21]. Available from: https://www.fda.gov/media/132674/download.
Wright CE, Sisson TL, Ichhpurani AK, Peters GR. Steady-state pharmacokinetic properties of pramipexole in healthy volunteers. J Clin Pharmacol. 1997;37(6):520–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saepang, K., Li, S.K. & Chantasart, D. Effect of Pulsed Direct Current on Iontophoretic Delivery of Pramipexole across Human Epidermal Membrane In Vitro. Pharm Res 38, 1187–1198 (2021). https://doi.org/10.1007/s11095-021-03055-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03055-3