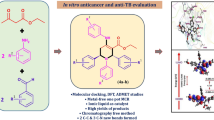
Condensation of 4-chloroacetophenone 1 with phenyl hydrazine 2 afforded hydrazone 3. Further reaction of 3 with Vilsmeier reagent yielded the pyrazolaldehyde 4 in excellent yield. A one-pot, three-component reaction between aldehyde 4, triphenylphosphite 5, and appropriate amines in the presence of lithium perchlorate as Lewis acid catalyst gave the corresponding α-amino phosphonates 7-12(a-d) in good yields. The chemical structures of all new compounds were established by IR, 1H NMR, and mass spectroscopy analysis. The anti-proliferative activity of the synthesized compounds against HCT-116, HepG2 and MCF-7 human cancer cells using the MTT assay was evaluated, and revealed higher anticancer activity when compared with reference drug doxorubicin. Among the tested compounds, pyrazole derivatives 4 and 9 exhibited the highest anticancer activity against breast (MCF-7), and colon (HCT-116) cancer cell lines with IC50 = 2.7 and 3.3 μM, respectively.



Similar content being viewed by others
References
M. Mamdouh, A. Mohammed, E. A. Tarik, et al., Phosphorus Sulfur, 193, 668 – 674 (2018); https://doi.org/10.1080/10426507.2018.1487969
Zita Radai and Gyorgy Kegievich, Molecules, 1493, 1 – 29 (2018); https://doi.org/10.3390/molecules23061493
U. Satish, R. Kiran, R. Ashok, et al., 20, 5552 – 5558 (2018); https://doi.org/10.1002/slct.201800798
Rebiha Damiche and Salah Chafaa, J. Mol. Struct., 1130, 1009 – 1017 (2017); https://doi.org/10.1016/j.molstruc.2016.10.054
Mohamed K. Awad, Mahmoud F. Abdel-Aal, Faten M. Atlam, and Hend A. Hekal, J. Mol. Struct., 1173, 128 – 141 (2018); https://doi.org/10.1016/j.molstruc.2018.06.094
F. L. Berenice, R. Misael, S. C. Rodrigo, O. M. Gabriela, Med. Chem., 27, 2376 – 2386 (2019); https://doi.org/10.1016/j.bmc.2018.12.041
S. T. Mohammed, M. Reda, S. Abdulrahman, et al., Int. J. Org. Chem., 8, 1 – 15 (2018); https://doi.org/10.4236/ijoc.2018.81001
M. Mohamed, S. Ahmed, A. Abeer, et al., J. Saudi Chem. Soc., 22, 34 – 41 (2018); https://doi.org/10.1016/j.jscs.2017.06.002
P. Van der Veken, I. El Sayed, J. Joossens, et al., Synthesis, 634 – 638 (2005).
E. Ramzy, A. G. Ahmed, A. Asem, et al., IJPSR. 5, 14 – 20 (2016).
I. El Sayed, S. El Kosy, F. Mohamed, et al., Am. J. Sci. 7, 604 – 608 (2011).
K. S. Kandula Madhu, S. Shaik Mahammad, P. Nagaripati, et al., Res. Chem. Intermediat., 43, 7087 – 7103 (2017); https://doi.org/10.1007/s11164-017-3060-y
J. Joossens, P. Van der Veken, A. Lambeir, et al., J. Med, 49, 5785 – 5793 (2006).
M. O. Ali, J. Joossens, I. El Sayed, et al., J. Med, 50, 6638 – 6646 (2007).
El B. Hanaa, A. G. Abdelaleem, I. El Sayed, and A. Mariam, J. Med., 24, 2142 – 21532 (2015).
E. A. Imam, I. El Sayed, M. G. Mahfouz, et al., Chem. Eng. 352, 1022 – 1034 (2018).
Z. Zi-Li, Li Jing, C. Hai-dong, et al., Chem. Pharm. Bull., 64, 1755 – 1762 (2016); doi:10.1248/cpb.c16-00622
I. El Sayed, A. Abdelaleem, M. S. Ibrahim, et al., IJPSR, 7, 181 – 189 (2016); https://doi.org/10.13040/IJPSR.0975-8232.7(1).181-89
A. S. Abdullah, A. Abdelaleem, A. M. Nayera, and I. El Sayed, Pharm. Sci. Rev. Res., 36, 35 – 39 (2016).
M. Hamed, A. Abdelaleem, A. Fathy, et al., Pharm. Sci. Rev. Res., 34, 215 – 213 (2015).
M. K. Salah, I. El Sayed, T. Aliaa, et al., Am. J. Sci., 7, 357 – 361 (2011).
S. H. Ashraf, F. M. Mohamed, M. Hanem, and S. Taghrid, Chin. Chem. Lett., 28, 388 – 393 (2017).
M. Eman, A. Wael, M. Ashraf, et al., Molecules, 22, 1 – 13 (2017); https://doi.org/doi.org/10.3390/molecules22010170
F. Salwa, R. Eman, A. Eman, et al., Res. Chem. Intermediat., 43, 437 – 456 (2017); https://doi.org/10.1007/s11164-016-2633-5
Acknowledgments
We would like to acknowledge financial support of the Menoufia University throughout project No Ib-2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelwahed, R.E., Radhi, A.H., Awad, H.M. et al. Synthesis and Anti-Proliferative Activity of New α-Amino Phosphonate Derivatives Bearing Heterocyclic Moiety. Pharm Chem J 55, 231–239 (2021). https://doi.org/10.1007/s11094-021-02404-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02404-1