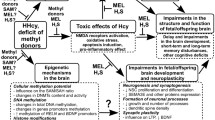
Abstract
The article presents current views on maternal hyperhomocysteinemia (HHcy) as an important factor causing prenatal stress and impaired nervous system development in fetuses and newborns in early ontogenesis, as well as complications in adulthood. Experimental data demonstrate that prenatal HHcy (PHHcy) affects the morphological maturation of the brain and activity of its neurotransmitter systems. Cognitive deficit observed in the offspring subjected to PHHcy in experimental studies can presumably cause the predisposition to various neurodegenerative diseases, as the role of maternal HHcy in the pathogenesis such diseases has been proven in clinical studies. The review also discusses molecular mechanisms of the HHcy neurotoxic action on the nervous system development in the prenatal and early postnatal periods, which include oxidative stress, apoptosis activation, changes in the DNA methylation patterns and microRNA levels, altered expression and processing of neurotrophins, and neuroinflammation induced by an increased production of pro-inflammatory cytokines. Special attention is given to the maternal HHcy impact on the placenta function and its possible contribution to the brain function impairments in the offspring. Published data suggest that some effects of PHHcy on the developing fetal brain can be due to the disturbances in the transport functions of the placenta resulting in an insufficient supply of nutrients necessary for the proper formation and functioning of brain structures.

Similar content being viewed by others
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- CBS:
-
cystathionine β-synthase
- CSE:
-
cystathionine γ-lyase
- E:
-
day of embryonic development
- F1:
-
first-generation progeny
- Hcy:
-
homocysteine
- HHcy:
-
hyperhomocysteinemia
- MTHFR:
-
methylenetetrahydrofolate reductase
- NGF:
-
nerve growth factor
- P:
-
day of postnatal development
- PHHcy:
-
prenatal hyperhomocysteinemia
- SAH:
-
S-adenosylhomocysteine
- SAM:
-
S-adenosylmethionine
References
Boersma, G. J., Bale, T. L., Casanello, P., Lara, H. E., Lucion, A. B., et al. (2014) Long-term impact of early life events on physiology and behaviour, J. Neuroendocrinol., 26, 587-602, https://doi.org/10.1111/jne.12153.
Entringer, S., Buss, C., and Wadhwa, P. D. (2015) Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper, Psychoneuroendocrinology, 62, 366-75, https://doi.org/10.1016/j.psyneuen.2015.08.019.
Bolton, J. L., and Bilbo, S. D. (2014) Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms, Dialogues Clin. Neurosci., 16, 307-320.
Buss, C., Entringer, S., and Wadhwa, P. D. (2012) Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology, Sci. Signal., 5, pt7, https://doi.org/10.1126/scisignal.2003406.
Cottrell, E. C., Seckl, J. R., Holmes, M. C., and Wyrwoll, C. S. (2014) Foetal and placental 11beta-HSD2: a hub for developmental programming, Acta Physiol. (Oxf), 210, 288-95, https://doi.org/10.1111/apha.12187.
Marques, A. H., Bjorke-Monsen, A. L., Teixeira, A. L., and Silverman, M. N. (2015) Maternal stress, nutrition and physical activity: impact on immune function, CNS development and psychopathology, Brain Res., 1617, 28-46, https://doi.org/10.1016/j.brainres.2014.10.051.
Williams, J. H., and Ross, L. (2007) Consequences of prenatal toxin exposure for mental health in children and adolescents: a systematic review, Eur. Child Adolesc. Psychiatry, 16, 243-53, https://doi.org/10.1007/s00787-006-0596-6.
Boldyrev, A. A. (2009) Molecular mechanisms of homocysteine toxicity, Biochemistry (Moscow), 74, 589-598, https://doi.org/10.1134/s0006297909060017.
Troen, A. M. (2005) The central nervous system in animal models of hyperhomocysteinemia, Prog. Neuropsychopharmacol. Biol. Psychiatry, 29, 1140-1151, https://doi.org/10.1016/j.pnpbp.2005.06.025.
Cascalheira, J. F., Parreira, M. C., Viegas, A. N., Faria, M. C., and Domingues, F. C. (2008) Serum homocysteine: relationship with circulating levels of cortisol and ascorbate, Ann. Nutr. Metab., 53, 67-74, https://doi.org/10.1159/000158636.
Stoney, C. M. (1999) Plasma homocysteine levels increase in women during psychological stress, Life Sci., 64, 2359-65, https://doi.org/10.1016/s0024-3205(99)00189-7.
Tallova, J., Bicikova, M., Hill, M., Tomandl, J., and Valentova, D. (2003) Homocysteine during the menstrual cycle in depressive women, Eur. J. Clin. Invest., 33, 268-273, https://doi.org/10.1046/j.1365-2362.2003.01087.x.
Zhao, Y., Wu, S., Gao, X., Zhang, Z., Gong, J., et al. (2013) Inhibition of cystathionine beta-synthase is associated with glucocorticoids over-secretion in psychological stress-induced hyperhomocysteinemia rat liver, Cell Stress Chaperones, 18, 631-641, https://doi.org/10.1007/s12192-013-0416-0.
Selhub, J. (1999) Homocysteine metabolism, Annu. Rev. Nutr., 19, 217-246, https://doi.org/10.1146/annurev.nutr.19.1.217.
Gueant, J. L., Namour, F., Gueant-Rodriguez, R. M., and Daval, J. L. (2013) Folate and fetal programming: a play in epigenomics? Trends Endocrinol. Metab., 24, 279-289, https://doi.org/10.1016/j.tem.2013.01.010.
Hannibal, L., and Blom, H. J. (2017) Homocysteine and disease: causal associations or epiphenomenons? Mol. Aspects Med., 53, 36-42, https://doi.org/10.1016/j.mam.2016.11.003.
James, S. J., Melnyk, S., Pogribna, M., Pogribny, I. P., and Caudill, M. A. (2002) Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology, J. Nutr., 132, 2361S-2366S, https://doi.org/10.1093/jn/132.8.2361S.
Yang, Q., and He, G. W. (2019) Imbalance of homocysteine and H2S: significance, mechanisms, and therapeutic promise in vascular injury, Oxid. Med. Cell Longev., 2019, 7629673, https://doi.org/10.1155/2019/7629673.
Skovierova, H., Vidomanova, E., Mahmood, S., Sopkova, J., Drgova, A., et al. (2016) The molecular and cellular effect of homocysteine metabolism imbalance on human health, Int. J. Mol. Sci., 17, https://doi.org/10.3390/ijms17101733.
Vitvitsky, V., Thomas, M., Ghorpade, A., Gendelman, H. E., and Banerjee, R. (2006) A functional transsulfuration pathway in the brain links to glutathione homeostasis, J. Biol. Chem., 281, 35785-35793, https://doi.org/10.1074/jbc.M602799200.
Kamat, P. K., Kyles, P., Kalani, A., and Tyagi, N. (2016) Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder, Mol. Neurobiol., 53, 2451-2467, https://doi.org/10.1007/s12035-015-9212-4.
Kumar, M., Modi, M., and Sandhir, R. (2017) Hydrogen sulfide attenuates homocysteine-induced cognitive deficits and neurochemical alterations by improving endogenous hydrogen sulfide levels, Biofactors, 43, 434-450, https://doi.org/10.1002/biof.1354.
Kumar, M., and Sandhir, R. (2019) Hydrogen sulfide suppresses homocysteine-induced glial activation and inflammatory response, Nitric Oxide, 90, 15-28, https://doi.org/10.1016/j.niox.2019.05.008.
Patel, D., Rathinam, M., Jarvis, C., Mahimainathan, L., Henderson, G., et al. (2018) Role for cystathionine gamma lyase (CSE) in an ethanol (E)-induced lesion in fetal brain GSH homeostasis, Int. J. Mol. Sci., 19, https://doi.org/10.3390/ijms19051537.
Borowczyk, K., Shih, D. M., and Jakubowski, H. (2012) Metabolism and neurotoxicity of homocysteine thiolactone in mice: evidence for a protective role of paraoxonase 1, J. Alzheimer’s Dis., 30, 225-231, https://doi.org/10.3233/JAD-2012-111940.
Kamudhamas, A., Pang, L., Smith, S. D., Sadovsky, Y., and Nelson, D. M. (2004) Homocysteine thiolactone induces apoptosis in cultured human trophoblasts: a mechanism for homocysteine-mediated placental dysfunction? Am. J. Obstet. Gynecol., 191, 563-571, https://doi.org/10.1016/j.ajog.2004.01.037.
Perla-Kajan, J., and Jakubowski, H. (2012) Paraoxonase 1 and homocysteine metabolism, Amino Acids, 43, 1405-1417, https://doi.org/10.1007/s00726-012-1321-z.
Sharma, G. S., Kumar, T., Dar, T. A., and Singh, L. R. (2015) Protein N-homocysteinylation: from cellular toxicity to neurodegeneration, Biochim. Biophys. Acta, 1850, 2239-45, https://doi.org/10.1016/j.bbagen.2015.08.013.
Herrmann, W., and Obeid, R. (2011) Homocysteine: a biomarker in neurodegenerative diseases, Clin. Chem. Lab. Med., 49, 435-441, https://doi.org/10.1515/CCLM.2011.084.
Sharma, M., Tiwari, M., and Tiwari, R. K. (2015) Hyperhomocysteinemia: impact on neurodegenerative diseases, Basic Clin. Pharmacol. Toxicol., 117, 287-296, https://doi.org/10.1111/bcpt.12424.
Kamat, P. K., Vacek, J. C., Kalani, A., and Tyagi, N. (2015) Homocysteine induced cerebrovascular dysfunction: a link to Alzheimer’s disease etiology, Open Neurol. J., 9, 9-14, https://doi.org/10.2174/1874205X01509010009.
Azzini, E., Ruggeri, S., and Polito, A. (2020) Homocysteine: Its possible emerging role in at-risk population groups, Int. J. Mol. Sci., 21, https://doi.org/10.3390/ijms21041421.
Zhuo, J. M., Wang, H., and Pratico, D. (2011) Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol. Sci., 32, 562-571, https://doi.org/10.1016/j.tips.2011.05.003.
Weekman, E. M., Sudduth, T. L., Price, B. R., Woolums, A. E., Hawthorne, D., et al. (2019) Time course of neuropathological events in hyperhomocysteinemic amyloid depositing mice reveals early neuroinflammatory changes that precede amyloid changes and cerebrovascular events, J. Neuroinflammation, 16, 284, https://doi.org/10.1186/s12974-019-1685-z.
Degroote, S., Hunting, D., and Takser, L. (2018) Periconceptional folate deficiency leads to autism-like traits in Wistar rat offspring, Neurotoxicol. Teratol., 66, 132-138, https://doi.org/10.1016/j.ntt.2017.12.008.
Jozefczuk, J., Kasprzycka, W., Czarnecki, R., Graczyk, A., Jozefczuk, P., et al. (2017) Homocysteine as a diagnostic and etiopathogenic factor in children with autism spectrum disorder, J. Med. Food, 20, 744-749, https://doi.org/10.1089/jmf.2016.0150.
James, S. J., Melnyk, S., Jernigan, S., Pavliv, O., Trusty, T., et al. (2010) A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism, Am. J. Med. Genet. B Neuropsychiatr. Genet., 153B, 1209-1220, https://doi.org/10.1002/ajmg.b.31094.
Baydas, G., Koz, S. T., Tuzcu, M., Nedzvetsky, V. S., and Etem, E. (2007) Effects of maternal hyperhomocysteinemia induced by high methionine diet on the learning and memory performance in offspring, Int. J. Dev. Neurosci., 25, 133-139, https://doi.org/10.1016/j.ijdevneu.2007.03.001.
Koz, S. T., Gouwy, N. T., Demir, N., Nedzvetsky, V. S., Etem, E., and Baydas, G. (2010) Effects of maternal hyperhomocysteinemia induced by methionine intake on oxidative stress and apoptosis in pup rat brain, Int. J. Dev. Neurosci., 28, 325-329, https://doi.org/10.1016/j.ijdevneu.2010.02.006.
Blaise, S. A., Nedelec, E., Schroeder, H., Alberto, J. M., Bossenmeyer-Pourie, C., et al. (2007) Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats, Am. J. Pathol., 170, 667-679, https://doi.org/10.2353/ajpath.2007.060339.
Arutjunyan, A., Kozina, L., Stvolinskiy, S., Bulygina, Y., Mashkina, A., and Khavinson, V. (2012) Pinealon protects the rat offspring from prenatal hyperhomocysteinemia, Int. J. Clin. Exp. Med., 5, 179-185.
Shcherbitskaya, A. D., Milyutina, Y. P., Zaloznyaya, I. V., Arutjunyan, A. V., Nalivaeva, N. N., and Zhuravin, I. A. (2017) The effects of prenatal hyperhomocysteinemia on the formation of memory and the contents of biogenic amines in the rat hippocampus, Neurochem. J., 11, 296-301, https://doi.org/10.1134/s1819712417040080.
Ars, C. L., Nijs, I. M., Marroun, H. E., Muetzel, R., Schmidt, M., et al. (2019) Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study, Br. J. Nutr., 122, S1-S9, https://doi.org/10.1017/s0007114515002081.
Yakovleva, O. V., Ziganshina, A. R., Dmitrieva, S. A., Arslanova, A. N., Yakovlev, A. V., et al. (2018) Hydrogen sulfide ameliorates developmental impairments of rat offspring with prenatal hyperhomocysteinemia, Oxid. Med. Cell. Longev., 2018, 2746873, https://doi.org/10.1155/2018/2746873.
Yakovleva, O. V., Ziganshina, A. R., Gerasimova, E. V., Arslanova, A. N., Yarmiev, I. Z., et al. (2019) Influence of group B vitamins on the early development of pup rats with prenatal hyperhomocysteinemia, Sechenov Ros. Fiziologich. Zhurn., 105, 1247-1261, https://doi.org/10.1134/S086981391910011X.
Jadavji, N. M., Deng, L., Malysheva, O., Caudill, M. A., and Rozen, R. (2015) MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring, Neuroscience, 300, 1-9, https://doi.org/10.1016/j.neuroscience.2015.04.067.
Geoffroy, A., Saber-Cherif, L., Pourie, G., Helle, D., Umoret, R., et al. (2019) Developmental impairments in a rat model of methyl donor deficiency: effects of a late maternal supplementation with folic acid, Int. J. Mol. Sci., 20, https://doi.org/10.3390/ijms20040973.
Hassan, Z., Coelho, D., Kokten, T., Alberto, J. M., Umoret, R., et al. (2019) Brain susceptibility to methyl donor deficiency: from fetal programming to aging outcome in rats, Int. J. Mol. Sci., 20, https://doi.org/10.3390/ijms20225692.
Baydas, G., Koz, S. T., Tuzcu, M., and Nedzvetsky, V. S. (2008) Melatonin prevents gestational hyperhomocysteinemia-associated alterations in neurobehavioral developments in rats, J. Pineal Res., 44, 181-188, https://doi.org/10.1111/j.1600-079X.2007.00506.x.
Figueiro, P. W., de Moreira, D. S., Dos Santos, T. M., Prezzi, C. A., Rohden, F., et al. (2019) The neuroprotective role of melatonin in a gestational hypermethioninemia model, Int. J. Dev. Neurosci., 78, 198-209, https://doi.org/10.1016/j.ijdevneu.2019.08.004.
Schweinberger, B. M., Rodrigues, A. F., Dos Santos, T. M., Rohden, F., Barbosa, S., et al. (2018) Methionine administration in pregnant rats causes memory deficit in the offspring and alters ultrastructure in brain tissue, Neurotox. Res., 33, 239-246, https://doi.org/10.1007/s12640-017-9830-x.
Yakovleva, O., Bogatova, K., Mukhtarova, R., Yakovlev, A., Shakhmatova, V., et al. (2020) Hydrogen sulfide alleviates anxiety, motor, and cognitive dysfunctions in rats with maternal hyperhomocysteinemia via mitigation of oxidative stress, Biomolecules, 10, https://doi.org/10.3390/biom10070995.
Makhro, A. V., Mashkina, A. P., Solenaya, O. A., Trunova, O. A., Kozina, L. S., et al. (2008) Prenatal hyperhomocysteinemia as a model of oxidative stress of the brain, Bull. Exp. Biol. Med., 146, 33-35, https://doi.org/10.1007/s10517-008-0233-0.
Berrocal-Zaragoza, M. I., Sequeira, J. M., Murphy, M. M., Fernandez-Ballart, J. D., Abdel Baki, S. G., et al. (2014) Folate deficiency in rat pups during weaning causes learning and memory deficits, Br. J. Nutr., 112, 1323-1332, https://doi.org/10.1017/S0007114514002116.
Pourie, G., Martin, N., Daval, J. L., Alberto, J. M., Umoret, R., Gueant, J. L., and Bossenmeyer-Pourie, C. (2020) The stimulation of neurogenesis improves the cognitive status of aging rats subjected to gestational and perinatal deficiency of B9-12 vitamins, Int. J. Mol. Sci., 21, https://doi.org/10.3390/ijms21218008.
Pustygina, A. V., Milyutina, Y. P., Zaloznyaya, I. V., and Arutyunyan, A. V. (2015) Indices of oxidative stress in the brain of newborn rats subjected to prenatal hyperhomocysteinemia, Neurochem. J., 9, 60-65, https://doi.org/10.1134/s1819712415010079.
Dennery, P. A. (2010) Oxidative stress in development: nature or nurture? Free Radic. Biol. Med., 49, 1147-1151, https://doi.org/10.1016/j.freeradbiomed.2010.07.011.
Shcherbitskaia, A., Milyutina, Y., Zalozniaia, I., Kerkeshko, G., and Arutjunyan, A. (2020) Experimental hyperhomocysteinemia initiates oxidative stress in the mother-placenta-fetus system, Eur. J. Clin. Invest., 50, 52-53.
Schweinberger, B. M., Schwieder, L., Scherer, E., Sitta, A., Vargas, C. R., and Wyse, A. T. (2014) Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+,K+-ATPase/Mg2+-ATPase activity and oxidative status of the offspring, Metab. Brain Dis., 29, 153-60, https://doi.org/10.1007/s11011-013-9451-x.
Makhro, A. V., Mashkina, A. P., Solenaya, O. A., Tyulina, O. V., Bulygina, E. R., et al. (2008) Carnosine protects cells from oxidative stress induced by hyperhomocysteinemia, Neurochem. J., 2, 202-208, https://doi.org/10.1134/S1819712408030112.
Baydas, G., Ozer, M., Yasar, A., Koz, S. T., and Tuzcu, M. (2006) Melatonin prevents oxidative stress and inhibits reactive gliosis induced by hyperhomocysteinemia in rats, Biochemistry (Moscow), 71 Suppl 1, S91-S95, https://doi.org/10.1134/s0006297906130153.
Abushik, P. A., Niittykoski, M., Giniatullina, R., Shakirzyanova, A., Bart, G., et al. (2014) The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells, J. Neurochem., 129, 264-274, https://doi.org/10.1111/jnc.12615.
Li, W., Li, Z., Zhou, D., Zhang, X., Yan, J., and Huang, G. (2019) Maternal folic acid deficiency stimulates neural cell apoptosis via miR-34a associated with Bcl-2 in the rat foetal brain, Int. J. Dev. Neurosci., 72, 6-12, https://doi.org/10.1016/j.ijdevneu.2018.11.002.
Arutjunyan, A. V., Milyutina, Y. P., Shcherbitskaia, A. D., Kerkeshko, G. O., Zalozniaia, I. V., and Mikhel, A. V. (2020) Neurotrophins of the fetal brain and placenta in prenatal hyperhomocysteinemia, Biochemistry (Moscow), 85, 248-259, https://doi.org/10.1134/S000629792002008X.
Blaise, S. A., Nedelec, E., Alberto, J. M., Schroeder, H., Audonnet, S., et al. (2009) Short hypoxia could attenuate the adverse effects of hyperhomocysteinemia on the developing rat brain by inducing neurogenesis, Exp. Neurol., 216, 231-238, https://doi.org/10.1016/j.expneurol.2008.11.020.
Shcherbitskaia, A. D., Vasilev, D. S., Milyutina, Y. P., Tumanova, N. L., Zalozniaia, I. V., et al. (2020) Maternal hyperhomocysteinemia induces neuroinflammation and neuronal death in the rat offspring cortex, Neurotox. Res., 38, 408-420, https://doi.org/10.1007/s12640-020-00233-w.
Hsiao, E. Y., and Patterson, P. H. (2012) Placental regulation of maternal-fetal interactions and brain development, Dev. Neurobiol., 72, 1317-1326, https://doi.org/10.1002/dneu.22045.
Bale, T. L., Baram, T. Z., Brown, A. S., Goldstein, J. M., Insel, T. R., et al. (2010) Early life programming and neurodevelopmental disorders, Biol. Psychiatry, 68, 314-319, https://doi.org/10.1016/j.biopsych.2010.05.028.
Da Cunha, A. A., Ferreira, A. G., Loureiro, S. O., da Cunha, M. J., Schmitz, F., et al. (2012) Chronic hyperhomocysteinemia increases inflammatory markers in hippocampus and serum of rats, Neurochem. Res., 37, 1660-1669, https://doi.org/10.1007/s11064-012-0769-2.
Kim, K. C., Friso, S., and Choi, S. W. (2009) DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging, J. Nutr. Biochem., 20, 917-926, https://doi.org/10.1016/j.jnutbio.2009.06.008.
Liu, H. Y., Liu, S. M., and Zhang, Y. Z. (2020) Maternal folic acid supplementation mediates offspring health via DNA methylation, Reprod. Sci., 27, 963-976, https://doi.org/10.1007/s43032-020-00161-2.
Harlan De Crescenzo, A., Panoutsopoulos, A. A., Tat, L., Schaaf, Z., Racherla, S., et al. (2020) Deficient or excess folic acid supply during pregnancy alter cortical neurodevelopment in mouse offspring, Cereb. Cortex, https://doi.org/10.1093/cercor/bhaa248.
Geoffroy, A., Kerek, R., Pourie, G., Helle, D., Gueant, J. L., et al. (2017) Late maternal folate supplementation rescues from methyl donor deficiency-associated brain defects by restoring Let-7 and miR-34 pathways, Mol. Neurobiol., 54, 5017-5033, https://doi.org/10.1007/s12035-016-0035-8.
Li, J. G., Barrero, C., Gupta, S., Kruger, W. D., Merali, S., and Pratico, D. (2017) Homocysteine modulates 5-lipoxygenase expression level via DNA methylation, Aging Cell, 16, 273-280, https://doi.org/10.1111/acel.12550.
Pogribny, I. P., Karpf, A. R., James, S. R., Melnyk, S., Han, T., and Tryndyak, V. P. (2008) Epigenetic alterations in the brains of Fisher 344 rats induced by long-term administration of folate/methyl-deficient diet, Brain Res., 1237, 25-34, https://doi.org/10.1016/j.brainres.2008.07.077.
Kalani, A., Kamat, P. K., Familtseva, A., Chaturvedi, P., Muradashvili, N., et al. (2014) Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism, J. Cereb. Blood Flow Metab., 34, 1212-1222, https://doi.org/10.1038/jcbfm.2014.74.
Kalani, A., Kamat, P. K., Givvimani, S., Brown, K., Metreveli, N., et al. (2014) Nutri-epigenetics ameliorates blood-brain barrier damage and neurodegeneration in hyperhomocysteinemia: role of folic acid, J. Mol. Neurosci., 52, 202-215, https://doi.org/10.1007/s12031-013-0122-5.
Langie, S. A., Achterfeldt, S., Gorniak, J. P., Halley-Hogg, K. J., Oxley, D., et al. (2013) Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring, FASEB J., 27, 3323-3334, https://doi.org/10.1096/fj.12-224121.
Sahay, A., Kale, A., and Joshi, S. (2020) Role of neurotrophins in pregnancy and offspring brain development, Neuropeptides, 102075, https://doi.org/10.1016/j.npep.2020.102075.
Parrish, R. R., Buckingham, S. C., Mascia, K. L., Johnson, J. J., Matyjasik, M. M., et al. (2015) Methionine increases BDNF DNA methylation and improves memory in epilepsy, Ann. Clin. Transl. Neurol., 2, 401-416, https://doi.org/10.1002/acn3.183.
Yan, Z., Jiao, F., Yan, X., and Ou, H. (2017) Maternal chronic folate supplementation ameliorates behavior disorders induced by prenatal high-fat diet through methylation alteration of BDNF and Grin2b in offspring hippocampus, Mol. Nutr. Food Res., 61, https://doi.org/10.1002/mnfr.201700461.
Yang, J., Harte-Hargrove, L. C., Siao, C. J., Marinic, T., Clarke, R., et al. (2014) proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus, Cell Rep., 7, 796-806, https://doi.org/10.1016/j.celrep.2014.03.040.
Gerenu, G., Martisova, E., Ferrero, H., Carracedo, M., Rantamaki, T., et al. (2017) Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits, Biochim. Biophys. Acta Mol. Basis Dis., 1863, 991-1001, https://doi.org/10.1016/j.bbadis.2017.01.023.
Schweinberger, B. M., Rodrigues, A. F., Turcatel, E., Pierozan, P., Pettenuzzo, L. F., et al. (2018) Maternal hypermethioninemia affects neurons number, neurotrophins levels, energy metabolism, and Na+,K+-ATPase expression/content in brain of rat offspring, Mol. Neurobiol., 55, 980-988, https://doi.org/10.1007/s12035-017-0383-z.
Canever, L., Freire, T. G., Mastella, G. A., Damazio, L., Gomes, S., et al. (2018) Changes in behavioural parameters, oxidative stress and neurotrophins in the brain of adult offspring induced to an animal model of schizophrenia: the effects of FA deficient or FA supplemented diet during the neurodevelopmental phase, Prog. Neuropsychopharmacol. Biol. Psychiatry, 86, 52-64, https://doi.org/10.1016/j.pnpbp.2018.05.014.
Bahous, R. H., Jadavji, N. M., Deng, L., Cosin-Tomas, M., Lu, J., et al. (2017) High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring, Hum. Mol. Genet., 26, 888-900, https://doi.org/10.1093/hmg/ddx004.
Shcherbitskaya, A. D., Milyutina, Y. P., Vasil’ev, D. S., Nalivaeva, N. N., Zhuravin, I. A., and Arutyunyan, A. V. (2020) Specific features of metabolism of biogenic amines in the hippocampus and adrenals of rats after prenatal hyperhomocysteinemia, Zhurn. Evol. Biokhim. Fiziol., 56, 724.
Kronenberg, G., Harms, C., Sobol, R. W., Cardozo-Pelaez, F., Linhart, H., et al. (2008) Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase, J. Neurosci., 28, 7219-7230, https://doi.org/10.1523/jneurosci.0940-08.2008.
Milyutina, Y. P., Arutyunyan, A. V., Pustygina, A. V., Shcherbitskaya, A. D., Zaloznyaya, I. V., and Zorina, I. I. (2014) Catecholamine levels in the adrenals of pup rat pups which underwent prenatal hyperhomocysteinemia, Sechenov Ross. Fiziol. Zhurn., 100, 360-369.
Arutyunyan, A. V., Zaloznyaya, I. V., Kerkeshko, G. O., Milyutina, Y. P., and Korenevskii, A. V. (2017) Prenatal hyperhomocysteinemia impairs hypothalamic regulation of reproductive cycles in rat progeny, Bull. Exp. Biol. Med., 162, 738-740, https://doi.org/10.1007/S10517-017-3701-6.
Saber Cherif, L., Pourie, G., Geoffroy, A., Julien, A., Helle, D., et al. (2019) Methyl donor deficiency during gestation and lactation in the rat affects the expression of neuropeptides and related receptors in the hypothalamus, Int. J. Mol. Sci., 20, https://doi.org/10.3390/ijms20205097.
Tsitsiou, E., Sibley, C. P., D’Souza, S. W., Catanescu, O., Jacobsen, D. W., and Glazier, J. D. (2011) Homocysteine is transported by the microvillous plasma membrane of human placenta, J. Inherit. Metab. Dis., 34, 57-65, https://doi.org/10.1007/s10545-010-9141-3.
Di Simone, N., Maggiano, N., Caliandro, D., Riccardi, P., Evangelista, A., et al. (2003) Homocysteine induces trophoblast cell death with apoptotic features, Biol. Reprod., 69, 1129-1134, https://doi.org/10.1095/biolreprod.103.015800.
Di Simone, N., Riccardi, P., Maggiano, N., Piacentani, A., D’Asta, M., et al. (2004) Effect of folic acid on homocysteine-induced trophoblast apoptosis, Mol. Hum. Reprod., 10, 665-669, https://doi.org/10.1093/molehr/gah091.
Kasture, V. V., Sundrani, D. P., and Joshi, S. R. (2018) Maternal one carbon metabolism through increased oxidative stress and disturbed angiogenesis can influence placental apoptosis in preeclampsia, Life Sci., 206, 61-69, https://doi.org/10.1016/j.lfs.2018.05.029.
Kim, J. M., Hong, K., Lee, J. H., Lee, S., and Chang, N. (2009) Effect of folate deficiency on placental DNA methylation in hyperhomocysteinemic rats, J. Nutr. Biochem., 20, 172-176, https://doi.org/10.1016/j.jnutbio.2008.01.010.
Li, B., Chang, S., Liu, C., Zhang, M., Zhang, L., et al. (2019) Low maternal dietary folate alters retrotranspose by methylation regulation in intrauterine growth retardation (IUGR) fetuses in a mouse model, Med. Sci. Monit., 25, 3354-3365, https://doi.org/10.12659/MSM.914292.
McGee, M., Bainbridge, S., and Fontaine-Bisson, B. (2018) A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes, Nutr. Rev., 76, 469-478, https://doi.org/10.1093/nutrit/nuy006.
Park, B. H., Kim, Y. J., Park, J. S., Lee, H. Y., Ha, E. H., et al. (2005) [Folate and homocysteine levels during pregnancy affect DNA methylation in human placenta], J. Prev. Med. Public Health, 38, 437-442.
Mahajan, A., Sapehia, D., Thakur, S., Mohanraj, P. S., Bagga, R., and Kaur, J. (2019) Effect of imbalance in folate and vitamin B12 in maternal/parental diet on global methylation and regulatory miRNAs, Sci. Rep., 9, 17602, https://doi.org/10.1038/s41598-019-54070-9.
Li, Y., Gao, R., Liu, X., Chen, X., Liao, X., et al. (2015) Folate deficiency could restrain decidual angiogenesis in pregnant mice, Nutrients, 7, 6425-6445, https://doi.org/10.3390/nu7085284.
Oosterbaan, A. M., Steegers, E. A., and Ursem, N. T. (2012) The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development, Microvasc. Res., 83, 98-104, https://doi.org/10.1016/j.mvr.2011.11.001.
Lai, W. K., and Kan, M. Y. (2015) Homocysteine-induced endothelial dysfunction, Ann. Nutr. Metab., 67, 1-12, https://doi.org/10.1159/000437098.
Chen, Y. Y., Gupta, M. B., Grattton, R., Powell, T. L., and Jansson, T. (2018) Down-regulation of placental folate transporters in intrauterine growth restriction, J. Nutr. Biochem., 59, 136-141, https://doi.org/10.1016/j.jnutbio.2018.06.003.
Hague, W. M. (2003) Homocysteine and pregnancy, Best Pract. Res. Clin. Obstet. Gynaecol., 17, 459-469.
Gaiday, A. N., Tussupkaliyev, A. B., Bermagambetova, S. K., Zhumagulova, S. S., Sarsembayeva, L. K., et al. (2018) Effect of homocysteine on pregnancy: a systematic review, Chem. Biol. Interact., 293, 70-76, https://doi.org/10.1016/j.cbi.2018.07.021.
Burton, G. J., and Jauniaux, E. (2018) Pathophysiology of placental-derived fetal growth restriction, Am. J. Obstet. Gynecol., 218, S745-S761, https://doi.org/10.1016/j.ajog.2017.11.577.
Burton, G. J., Redman, C. W., Roberts, J. M., and Moffett, A. (2019) Pre-eclampsia: pathophysiology and clinical implications, BMJ, 366, l2381, https://doi.org/10.1136/bmj.l2381.
Eldibany, M. M., and Caprini, J. A. (2007) Hyperhomocysteinemia and thrombosis: an overview, Arch. Pathol. Lab. Med., 131, 872-884, https://doi.org/10.1043/1543-2165(2007)131[872:HATAO]2.0.CO;2.
Harpel, P. C., Zhang, X., and Borth, W. (1996) Homocysteine and hemostasis: pathogenic mechanisms predisposing to thrombosis, J. Nutr., 126, 1285S-1289S, https://doi.org/10.1093/jn/126.suppl_4.1285S.
Van der Molen, E. F., Verbruggen, B., Novakova, I., Eskes, T. K., Monnens, L. A., and Blom, H. J. (2000) Hyperhomocysteinemia and other thrombotic risk factors in women with placental vasculopathy, BJOG, 107, 785-91, https://doi.org/10.1111/j.1471-0528.2000.tb13341.x.
Sahay, A. S., Sundrani, D. P., and Joshi, S. R. (2017) Neurotrophins: role in placental growth and development, Vitam. Horm., 104, 243-261, https://doi.org/10.1016/bs.vh.2016.11.002.
Fujita, K., Tatsumi, K., Kondoh, E., Chigusa, Y., Mogami, H., et al. (2011) Differential expression and the anti-apoptotic effect of human placental neurotrophins and their receptors, Placenta, 32, 737-744, https://doi.org/10.1016/j.placenta.2011.07.001.
Kawamura, K., Kawamura, N., Sato, W., Fukuda, J., Kumagai, J., and Tanaka, T. (2009) Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival, Endocrinology, 150, 3774-3782, https://doi.org/10.1210/en.2009-0213.
Toti, P., Ciarmela, P., Florio, P., Volpi, N., Occhini, R., and Petraglia, F. (2006) Human placenta and fetal membranes express nerve growth factor mRNA and protein, J. Endocrinol. Invest., 29, 337-341, https://doi.org/10.1007/BF03344105.
Mayeur, S., Lukaszewski, M. A., Breton, C., Storme, L., Vieau, D., and Lesage, J. (2011) Do neurotrophins regulate the feto-placental development? Med. Hypotheses, 76, 726-728, https://doi.org/10.1016/j.mehy.2011.02.008.
Akahoshi, N., Yokoyama, A., Nagata, T., Miura, A., Kamata, S., and Ishii, I. (2019) Abnormal amino acid profiles of blood and cerebrospinal fluid from cystathionine beta-synthase-deficient mice, an animal model of homocystinuria, Biol. Pharm. Bull, 42, 1054-1057, https://doi.org/10.1248/bpb.b19-00127.
Jansson, T. (2009) Novel mechanism causing restricted fetal growth: does maternal homocysteine impair placental amino acid transport? J. Physiol., 587, 4123, https://doi.org/10.1113/jphysiol.2009.178327.
Tsitsiou, E., Sibley, C. P., D’Souza, S. W., Catanescu, O., Jacobsen, D. W., and Glazier, J. D. (2009) Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta, J. Physiol., 587, 4001-4013, https://doi.org/10.1113/jphysiol.2009.173393.
Mori, M., Yamashita, Y., Hiroi, Y., Shinjo, S., Asato, R., et al. (1999) Effect of single essential amino acid excess during pregnancy on dietary nitrogen utilization and fetal growth in rats, Asia Pac. J. Clin. Nutr., 8, 251-257, https://doi.org/10.1046/j.1440-6047.1999.00094.x.
Matsueda, S., and Niiyama, Y. (1982) The effects of excess amino acids on maintenance of pregnancy and fetal growth in rats, J. Nutr. Sci. Vitaminol. (Tokyo), 28, 557-73, https://doi.org/10.3177/jnsv.28.557.
Rees, W. D., Wilson, F. A., and Maloney, C. A. (2006) Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet, J. Nutr., 136, 1701S-1705S, https://doi.org/10.1093/jn/136.6.1701S.
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 18-015-00099) and by the State Task (AAAA-A19-119021290116-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest. This article does not describe any research involving humans or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Arutjunyan, A.V., Kerkeshko, G.O., Milyutina, Y.P. et al. Prenatal Stress in Maternal Hyperhomocysteinemia: Impairments in the Fetal Nervous System Development and Placental Function. Biochemistry Moscow 86, 716–728 (2021). https://doi.org/10.1134/S0006297921060092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921060092