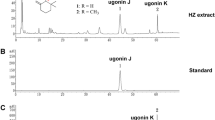
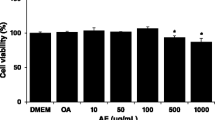
Abstract
While the underlying mechanism remains unknown, Rubus chingii var. suavissimus (S. K. Lee) L. T. Lu or Rubus suavissimus S. Lee (RS), a sweet plant distributed in southwest of China, has been used as beverage and folk medicine. Pharmacological studies indicated the potential of RS improving the obesity phenotype and hyperlipidemia. The mechanism is still not yet to be put forward. To verify the substantial effects of RS on lipid metabolism, a Syrian golden hamster model was adopted. The physiological and pathological evaluation of experimental animals demonstrated that RS can relieve the lipid metabolism disorder induced by high-fat diet and alleviated liver injury. RS upregulation the expressions of peroxisome proliferator-activated receptor α (PPARα), PPARγ and CCAAT/enhancer binding protein α (C/EBPα), as well as adipocyte-specific genes, glucose transporter 4 (Glut4), lipoprotein lipase (LPL) and fatty acid binding protein 4 (aP2). On the other side, RS suppressed the sterol regulatory element binding protein 1 (SREBP1) and downstream acetyl-CoA carboxylase 1 (ACC1), stearoyl-CoA desaturase-1 (SCD1) and fatty acid synthase (FAS). In conclusion, RS alleviated lipid metabolism disorder symptoms caused by high-fat diet accompanied with 8 weeks of treatment, involving enhanced β-oxidation, increased adipogenesis and decreased the metabolism of fatty acids, via modulation of the PPARs/SREBP pathway in Syrian golden hamsters.




Similar content being viewed by others
References
Li L, Yang GY (2007) Lipid dysmetabolism, adipocytokine and insulin resistance. Chin J Diab 15(3):129–131
Madsen MS, Siersbæk R, Boergesen M, Nielsen R, Mandrup S (2014) Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol Cell Biol 34(6):939–954. https://doi.org/10.1128/MCB.01344-13
Gregoire FM, Fau SC, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78(3):783–809. https://doi.org/10.1152/physrev.1998.78.3.783
Darlington GJ, Ross SF, MacDougald OA (1998) The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273(46):30057–30060. https://doi.org/10.1074/jbc.273.46.30057
Lin FT, Lane MD (1994) CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA 91(19):8757–8761. https://doi.org/10.1073/pnas.91.19.8757
Ding PP, Zhang LY (2019) The role of Peroxisome proliferators-activated receptors α in lipid metabolism. Chin J Clin Ration Drug Use. https://doi.org/10.15887/j.cnki.13-1389/r.2019.04.103
Wang J, Chen H, Sun X, Al E (2019) Research advances in peroxisome proliferator -activated receptor γ and related diseases. China Mod Med 26(12):36–38
Wang ZH, Dong MG, Chang XT, Hou XP (2005) Research progress of fatty acid binding protein and the formation mechanism of hyperlipidemia. Clin Med China 21(04):90–91
Horton JD, Goldstein JL, Brown MS (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109:1125–1131. https://doi.org/10.1172/JCI15593
Cui H, Chen LY, Wei HQ, Ning HX, Li YL (2019) Recearch progress on sterol regulatory element-binding proteins inhibitors. Drugs Clin 034(008):2560–2566. https://doi.org/10.7501/j.issn.1674-5515.2019.08.066
Guangxi Zhuang Autonomous Region Food and Drug Administration (2011) Quality Standard for Zhuang Medicine in Guangxi Zhuang Autonomous Region. Guangxi Science and Technology Press, China
Jeon TI, Osborne TF (2012) SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab Tem. https://doi.org/10.1016/j.tem.2011.10.004
Wen J, Tuo QH (2011) SREBP affects cell growth by regulating lipid metabolism. Chemistry of Life 31(06):790–795
Xie ZS, Zhao ZB, Zhang ZQ, Al E (2018) Research progress in pharmacology of natural small molecule inhibitors of SREBPs. China Pharm 29(10):1435–1440. https://doi.org/10.6039/j.issn.1001-0408.2018.10.34
Li SK (1981) Sweet tea, a new Rubus (Rosaceae) from Guangxi. Guihaia 1(4):17–19
Wei JF, Huang XC (1996) Identification of original tea plants in Guangxi. J Chin Med Mat 19(1):16–17
Ohtani K, Aikawa Y, Kasai R, Chou WH, Yamasaki K, Tanaka O (1992) Minor diterpene glycosides from sweet leaves of Rubus suavissimus. Phytochemistry 31(5):1553–1559. https://doi.org/10.1016/0031-9422(92)83105-8
Hirono S, Chou WH, Kasai R, Tanaka O, Tada TJC (1990) Sweet and bitter diterpene-glucosides from leaves of Rubus suavissimus. Chem Pharm Bull 38(6):1743–1744. https://doi.org/10.1248/cpb.38.1743
Zhang QY (2006) Study on rubusoside and other chemical constituents in leaves of Rubus Suavissimus S.Lee. Guangxi Normal University Master´s thesis
Chen QB (2005) Separation, purification and determinationof flavone aglycones from Rubus suavissimus. Forest Sci Technol 30(1):46–48
Liu J, Zhou X, Weng M, Wang S (2014) The study of pharmacodynamics and mechanism of polyphenols from Rubus suavissimus S. Lee. Pharm Clin Chin Mat Med 30(6):51–54
Nordentoft I, Jeppesen PB, Hong J, Abudula R, Hermansen K (2008) Isosteviol increases insulin sensitivity and changes gene expression of key insulin regulatory genes and transcription factors in islets of the diabetic KKAy mouse. Diabetes Obes Metab 10(10):939–949. https://doi.org/10.1111/j.1463-1326.2007.00836.x
Wang L, Wei Y, Ning C, Zhang M, Fan P, Lei D, Du J, Gale M, Ma Y, Yang Y (2019) Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr J 66(10):923–936. https://doi.org/10.1507/endocrj.EJ18-0467
Zhang Y, Wang M, Dong H, Yu X, Zhang J (2018) Anti-hypoglycemic and hepatocyte-protective effects of hyperoside from Zanthoxylum bungeanum leaves in mice with high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int J Mol Med 41(1):77–86. https://doi.org/10.3892/ijmm.2017.3211
Qin G, Ma J, Huang Q, Yin H, Han J, Li M, Deng Y, Wang B, Hassan W, Shang J (2018) Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-beta signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int J Mol Sci. https://doi.org/10.3390/ijms19124126
Kim HM, Kim Y, Lee ES, Huh JH, Chung CH (2018) Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 55–56:63–70. https://doi.org/10.1016/j.nut.2018.03.010
Wang LL, Zhang ZC, Hassan W, Li Y, Liu J, Shang J (2015) Amelioration of free fatty acid-induced fatty liver by quercetin-3-O-β-d-glucuronide through modulation of peroxisome proliferator-activated receptor-alpha/sterol regulatory element-binding protein-1c signaling. Hepatol Res 46:225–238. https://doi.org/10.1111/hepr.12557
Zhang MF, Shen YQ (2015) Research progress on lipidemic regulation and antiobesity effects of oleanolic acid and ursolic acid. Drug Eval Res 38(1):90–97. https://doi.org/10.7501/j.issn.1674-6376.2014.01.018
Holvoet P, Rull A, García-Heredia A, López-Sanromà S, Geeraert B, Joven J, Camps J (2015) Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: a transcriptomic and metabolomic study. Food Chem Toxicol 77:22–33. https://doi.org/10.1016/j.fct.2014.12.017
Zhang Z, Wang H, Jiao R, Peng C, Wong YM, Yeung VSY, Huang Y, Chen ZY (2009) Choosing hamsters but not rats as a model for studying plasma cholesterol-lowering activity of functional foods. Mol Nutr Food Res 53(7):921–930. https://doi.org/10.1002/mnfr.200800517
Décordé K, Teissèdre PL, Sutra T, Ventura E, Cristol JP, Rouanet JM (2009) Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res 53(5):659–666. https://doi.org/10.1002/mnfr.200800165
Ling L, Zhao SP, Cheng YC, Li YL (2003) Xuezhikang decreases serum lipoprotein(a) and C-reactive protein concentrations in patients with coronary heart disease. Clin Chem 49(8):1347–1352. https://doi.org/10.1373/49.8.1347
Guo F, Zi T, Liu L, Feng R, Sun C (2017) A 1H-NMR based metabolomics study of the intervention effect of mangiferin on hyperlipidemia hamsters induced by a high-fat diet. Food Funct 8(7):2455–2464. https://doi.org/10.1039/c7fo00081b
Lv Y, Xu JE (2013) Correlation between PPAR and lipid metabolism in diabetes. J Precis Med 28(03):277–279
Horton JD (2002) Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans 30:1091. https://doi.org/10.1042/bst0301091
Kotzka J, Knebel B, Avci H, Al E (2010) Phosphorylation of sterol regulatory element-binding protein (SREBP)-1a links growth hormone action to lipid metabolism in hepatocytes. Atherosclerosis 1(213):156–165. https://doi.org/10.1016/j.atherosclerosis.2010.08.046
Chen Q, Liu MY, Yu HY, Li J, Wang SJ, Zhang Y, Qiu F, Wang T (2018) Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med 72(3):655–666. https://doi.org/10.1007/s11418-018-1199-5
Padmanaban M, Gopal T, Rajendran AB, Lakshmi BS (2018) Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem Biol Interact 284:80–89. https://doi.org/10.1016/j.cbi.2018.02.011
Shchelkunova TA, Morozov IA, Rubtsov PM, Bobryshev YV, Sobenin IA, Orekhov AN, Andrianova IV, Smirnov AN, Aikawa E (2013) Lipid regulators during atherogenesis: expression of LXR, PPAR, and SREBP mRNA in the human aorta. PLoS ONE. https://doi.org/10.1371/journal.pone.0063374
Zhang Y, Cui Y, Wang XL, Shang X, Qi ZG, Xue J, Zhao X, Deng M, Xie ML (2015) PPARα/γ agonists and antagonists differently affect hepatic lipid metabolism, oxidative stress and inflammatory cytokine production in steatohepatitic rats. Cytokine 75(1):127–135. https://doi.org/10.1016/j.cyto.2015.05.031
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31660095 and 81903918), Guangxi Natural Science Foundation (2019JJA140644), First-class discipline of Chinese materia medica in Guangxi (2019XK117), Graduate Education Innovation Program Project (YCSZ2020011 and YCSZ20190028) from Guangxi University of Chinese Medicine, Guangxi Key Laboratory of Zhuang and Yao Ethnic Medicine (2014No.32).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11418_2021_1536_MOESM1_ESM.tif
Supplementary Fig. S1 RS improved body weight, food intake, energy intake as well as serum biochemical parameters and hepatic lipids of golden hamsters on a high-fat diet. (a) Body weight, (b) Food intake, (c) Energy intake. (TIF 195 KB)
11418_2021_1536_MOESM2_ESM.tif
Supplementary Fig. S2 Effects of RS on relative protein expressions measured by Western blot in golden hamsters. (a–j) PPARα, PPARγ, C/EBPα, aP2, Glut4, LPL, SREBP1, ACC1, FAS, and SCD1 by real-time PCR, β-actin was used as a loading control. Data are presented as means ± SD, n=3. * P <0.05, ** P <0.01 compared to HFD group; #P<0.05, ## P <0.01 compared to ND group. (TIF 351 KB)
Rights and permissions
About this article
Cite this article
Jiang, MJ., Li, L., Huang, WF. et al. Rubus chingii var. suavissimus alleviates high-fat diet-induced lipid metabolism disorder via modulation of the PPARs/SREBP pathway in Syrian golden hamsters. J Nat Med 75, 884–892 (2021). https://doi.org/10.1007/s11418-021-01536-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-021-01536-8