Abstract
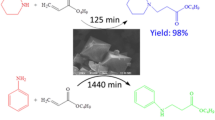
Copper-based metal–organic-frameworks with open metal sites have received increasing research interest as heterogeneous catalysts for various organic transformations. A copper-based metal organic framework (1) built with L-NO2 ligand (L-NO2 = 4,4′-dicarboxy-4″-nitrotriphenylamine) was selected for catalyzing aerobic homocoupling of arylboronic acid toward biaryl products given its structural robustness and 1-D channels lined with rich open metal sites. The experimental results show that MOF (1) exhibits pronounced size selectivity over arylboronic acid molecules, which is only effective for short arylboronic acid molecules (e.g. phenylboronic acid, p-methylphenylboronic acid and p-fluorophenylboronic acid), giving the corresponding biaryl products in good yields. Moreover, MOF (1) also demonstrates a good recyclability which only shows a small decay in the catalytic performance after five repeated runs.


Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this article [and its supplementary information files].
References
V. Pascanu, G.G. Miera, A.K. Inge, B. Martin-Matute, J. Am. Chem. Soc. 141, 7223 (2019)
A. Bavykina, N. Kolobov, I.S. Khan, J.A. Bau, A. Ramirez, J. Gascon, Chem. Rev. 120, 8468 (2020)
D.W. Bruce, D. Oare, R.I. Walton, Porous Materials (Wiley, Hoboken, 2010)
S. Subhadarshini, E. Pavitra, G.S.R. Raju, N.R. Chodankar, D.K. Goswami, Y.-K. Han, Y.S. Huh, N.C. Das, A.C.S. Appl, Mater. Interfaces 12, 29302 (2020)
S. Subhadarshini, E. Pavitra, G.S.R. Raju, N.R. Chodankar, A. Mandal, S. Roy, S. Mandal, M.V.B. Rao, D.K. Goswami, Y.S. Huh, N.C. Das, Ceram. Int. 47, 15293 (2021)
S. Subhadarshini, R. Singh, D.K. Goswami, A.K. Das, N.C. Das, Langmuir 35, 17166 (2019)
L. Jiao, J.Y.R. Seow, W.S. Skinner, S. William, Z.U. Wang, H.L. Jiang, Mat. Today. 27, 43 (2018)
T. Islamoglu, S. Goswami, Z.Y. Li, A.J. Howarth, O.K. Farha, J.T. Hupp, Account Chem. Res. 50, 805 (2017)
Y. Liao, W. Hung, T. Hou, C. Lin, K. Wong, Chem. Mater. 19, 6350 (2007)
J. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 102, 1359 (2002)
B. Yuan, Y. Pan, Y. Li, B. Yin, H. Jiang, Angew. Chem. Int. Ed. 49, 4054 (2010)
D. Lee, J.H. Kim, B.H. Jun, H. Kang, J. Parkand, Y.S. Lee, Org. Lett. 10, 1609 (2008)
A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.M. Basset, V. Polshettiwar, Chem. Soc. Rev. 40, 5181 (2011)
N.S.V. Stanley, J.S. Reis, I.M. de Oliveira, M.N. Balfour, H.A. Stefani, Tetrahedron 75, 1865 (2019)
M. Shimizua, I. Nagao, Y. Tomioka, T. Kadowaki, T. Hiyama, Tetrahedron 67, 8014 (2011)
S.Y. Xu, Y.B. Ruan, X.X. Luo, Y.F. Gao, J.S. Zhao, J.S. Shen, Y.B. Jiang, Chem. Commun. 46, 5864 (2010)
S.R. Cicco, G.M. Farinola, C. Martinelli, F. Naso, M. Tiecco, Eur. J. Org. Chem. 2010, 2275 (2010)
A. Prastaro, P. Ceci, E. Chiancone, A. Boffi, G. Fabrizi, S. Cacchi, Tetrahedron Lett. 51, 2250 (2010)
O. M. Yaghi, A. U. Czaja, B. Wang, Z. Lu, US Patents, 20120130113 (2012)
P. Puthiaraj, P. Suresh, K. Pitchumani, Green Chem. 16, 2865 (2014)
X.F. Yang, X.L. Chen, H.B. Zhu, Y. Shen, Polyhedron 157, 367 (2019)
S. Parshamoni, J. Telangae, S. Sanda, S. Konar, Chem. Asian J. 11, 540 (2016)
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (NSFC) (Grant No. 21171036), and the Fundamental Research Funds for the Central Universities (Grant No. 3207047406).
Funding
All funding information has been listed in the acknowledgement section.
Author information
Authors and Affiliations
Contributions
X-FY and L–LZ performed the experiment. H-BZ is responsible for data analysis and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
We have no any conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, XF., Zhang, LL. & Zhu, HB. Size-Selective Homocoupling of Arylboronic Acids Mediated by a Copper-Based Metal–Organic-Framework. J Inorg Organomet Polym 31, 4623–4627 (2021). https://doi.org/10.1007/s10904-021-02056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02056-4