Abstract
Daytime sleepiness is a cardinal symptom of obstructive sleep apnoea (OSA) and a well-recognised side effect of beta-blockers, therefore patients with OSA under this treatment may have worse sleepiness. However, the interaction between daytime sleepiness and beta-blockers use has not been thoroughly investigated in patients with OSA before. We analysed the data of 2183 individuals (1852 patients with OSA and 331 snorer controls) from 3 countries (Greece, Hungary and Moldova). Medical history, including medication usage and the Epworth Sleepiness Scale (ESS) were recorded. Patients and controls were divided into somnolent (ESS ≥ 11) and non-somnolent (ESS < 11) groups, and the association between-blocker use with the somnolent group was investigated with multivariate logistic regression analysis adjusted for confounders. Sensitivity analyses were performed in each cohort, in the severity subgroups, in patients who did not take statins and in those who had polysomnography as a diagnostic test. There was no relationship between beta-blocker usage and the somnolent OSA (p = 0.24) or control (p = 0.64) groups. These results were similar in sensitivity analyses (all p > 0.05). ESS was related to BMI (ρ = 0.25), total sleep time (ρ = 0.07), AHI (ρ = 0.32), oxygen desaturation index (ρ = 0.33) and minimum oxygen saturation (ρ = – 0.32, all p < 0.05) in OSA, and was higher in patients with hypertension, diabetes and cerebro/cardiovascular disease and those who took statins (all p < 0.05). In general, beta-blockers are not associated with increased daytime sleepiness in OSA. Thus, the diagnosis of OSA should not discourage initiation of beta-blocker treatment, if it is clinically indicated.
Similar content being viewed by others
Introduction
Obstructive sleep apnoea (OSA) is the most prevalent sleep-related breathing disorder. It is characterised by the repetitive collapse of the upper airways during sleep with consequential chronic intermittent hypoxia, frequent microarousals and activation of the sympathetic system. These lead to the increased prevalence of hypertension and cardiovascular disease in OSA [1].
Beta-receptor-blockers have been widely used in the therapy of numerous cardiovascular diseases, such as hypertension, ischaemic heart disease, and heart failure [2]. In addition, propranolol and metoprolol are also registered for the prevention of migraine due to their penetration to the central nervous system (CNS), while propranolol is frequently used to treat anxiety and thyrotoxicosis.
Beta-blockers have well-known potential CNS side effects which depend on their lipophilicity and consequential penetration through the blood–brain barrier [3]. In particular, sleep-related side effects include drowsiness, sleepiness, fatigue, nightmares and other parasomnias [3, 4]. Lipophilic drugs such as propranolol, metoprolol or betaxolol can enter CNS and achieve high concentration in the cerebrospinal fluid, while moderately lipophilic beta-blockers such as bisoprolol, nebivolol or carvedilol only partly penetrate through the blood–brain barrier [5]. In contrast, hydrophilic drugs such as sotalol or atenolol have a weak passage with poor distribution in CNS [6].
Excessive daytime sleepiness is a common symptom in patients with OSA. Epworth Sleepiness Scale (ESS), a self-rated standardised questionnaire assessing eight situations with the probability of falling asleep [7], is frequently used in clinical practice and in epidemiological studies to measure excessive daytime sleepiness [8].
As daytime sleepiness and cardiovascular diseases are common among patients with OSA, physicians may be reluctant to prescribe beta-blockers to avoid worsening sleepiness. However, the effect of beta-blockers on sleepiness in OSA has not been thoroughly investigated before. Therefore, the aim of the current study was to analyse beta-blocker use in three European OSA cohorts and to compare it with daytime sleepiness assessed with ESS.
Materials and methods
Subjects and design
Data of 1852 patients with OSA and 331 non-OSA snoring control subjects were analysed. The subjects participated in the Hungarian (n = 228 OSA and 84 controls), Greek (n = 1265 OSA and 247 controls) and Moldavian (n = 359 OSA) sleep apnoea cohorts. The volunteers were originally referred for a diagnostic sleep test due to symptoms suggestive of obstructive sleep apnoea (i.e. snoring, witnessed pauses in the breathing, daytime somnolence, tiredness, comorbidities, obesity, etc.). None of them had previously been diagnosed with OSA and they had not been treated with continuous positive airway pressure, mandibular advancement devices or upper airway surgery. Patients with acute heart failure, those who were diagnosed with central disorders of hypersomnolence or used any sedative drugs, such as antidepressant- and antipsychotic drugs, benzodiazepines, melatonin, or gamma-aminobutyric acid-agonists were excluded from analyses. Participants completed the valid translated version of ESS in their native language (Greek, Hungarian, Romanian, or Russian). This study was conducted in accordance with the Declaration of Helsinki. The studies have been approved by the local ethics committees (Semmelweis University TUKEB 30/2014, RKEB 172/2018, University General Hospital of Alexandroupolis 29/SB5/2014, State University of Medicine and Pharmacy "Nicolae Testemitanu" 39/44). All participants gave their written informed consent.
Sleep studies
Polysomnography and cardiorespiratory polygraphy were performed according to the American Academy of Sleep Medicine (AASM) recommendations [9]. Sleep stages and cardiorespiratory events were manually scored according to the AASM guidelines [10]. Total sleep time (TST) and minimal oxygen saturation (MinSatO2) were recorded, apnoea-hypopnoea index (AHI) and oxygen desaturation index (ODI) were calculated. In the OSA group AHI was ≥ 5/hour, criteria for the control group included AHI and ODI < 5/hour.
Statistical analysis
Statistica 12 (StatSoft, Inc., Tulsa, OK, US) and JASP 0.11.1 (University of Amsterdam, Amsterdam, Netherlands) were used for statistical analysis. Data normality was assessed with the Kolmogorov–Smirnov test. Variables were compared between the three cohorts, between patients with OSA and controls as well as with beta-blocker user and non-user patients with OSA with Chi-square tests, Fisher test, analysis of variance and Kruskal–Wallis test.
For the primary analysis, subjects were divided into somnolent (ESS ≥ 11) and non-somnolent (ESS < 11) groups and logistic regression analysis was used to assess their relationship with the beta-blocker use which was adjusted on age, gender, BMI, smoking, the presence of comorbidities (hypertension, diabetes, cerebro/cardiovascular disease, arrhythmia) and AHI. This analysis has been performed in patients with and without OSA, those patients who were not taking statins (n = 1415), those patients who had polysomnography as a diagnostic test and in each disease severity group separately. These analyses were also performed with each individual beta-blocker separately. For specific beta-blockers, patients on medication were compared to those who did not take any beta-blocker.
In addition, the bivariate relationships between ESS value and clinical variables were investigated with the Spearman test. The relationship between ESS value and beta-blocker use was analysed with non-parametric analysis of covariance (ANCOVA) where beta-blocker usage was used as a fixed factor and age, gender, body mass index (BMI), smoking, comorbidities and AHI were used as covariates. We also analysed the relationship between AHI and beta-blocker use with non-parametric ANCOVA which was adjusted on age, gender, BMI. Data are expressed as median /interquartile range/. A p value < 0.05 was considered significant.
Results
Subjects’ characteristics
There were significant differences in age, gender distribution, BMI, ESS, smoking history, the prevalence of comorbidities, beta-blocker use and sleep indices between the three cohorts of patients with OSA (Table 1). In controls, significant differences were noted in BMI, ESS, smoking history, the prevalence of hypertension, bisoprolol use, and sleep indices (Table 2).
Comparing patients with sleep apnoea to the controls, the patients were older, had higher BMI, ESS, AHI, ODI and lower MinSatO2. The prevalence rates of males, smokers, patients with hypertension, diabetes, cerebrovascular and cardiovascular disease, as well as statin and beta-blocker users were also higher in OSA (all p < 0.01). The proportion of patients taking bisoprolol was higher in OSA than controls (p < 0.01), but there was no difference in the prevalence of any other beta-blocker usage (all p > 0.05, Table 3).
Patients with OSA who used beta-blockers were older, had higher BMI, had a greater number of comorbidities, more prevalent statin usage and they also had more severe OSA (all p < 0.05, Table 4). Similarly, controls who took beta-blockers were older, had higher BMI, had a greater number of comorbidities, a more prevalent statin usage and longer TST (all p < 0.05, Table 5).
The association between the “somnolent” phenotype and beta-blockers use in patients with OSA and controls
Beta-blocker usage was not related to the somnolent phenotype in patients with OSA (p = 0.24) or controls (p = 0.64). The somnolent phenotype was not related to beta-blocker use in the subgroup of patients with OSA who did not take statins (p = 0.17) and those who had polysomnography as a diagnostic test (p = 0.29). No relationship was seen in either the Greek (p = 0.36), Hungarian (p = 0.30) or Moldavian OSA (p = 0.79), or Greek (p = 0.44) or Hungarian control (p = 0.75) groups.
There was no association between the somnolent phenotype and beta-blocker usage either in the mild group (pooled analysis p = 0.67; Greek cohort p = 0.81; Hungarian cohort p = 0.62, Moldavian cohort p = 0.97), in the moderate (pooled analysis p = 0.51; Greek cohort p = 0.24; Hungarian cohort p = 0.37, Moldavian cohort p = 0.91) or severe subgroups (pooled analysis p = 0.49; Greek cohort p = 0.86; Hungarian cohort p = 0.24, Moldavian cohort p = 0.85, Please see Table S1).
None of the beta-blockers were significantly associated with the somnolent phenotype either in patients with OSA (p = 0.12, p = 0.08, p = 0.27, p = 0.27, p = 0.57, p = 0.35, p = 0.35, p = 0.20 for bisoprolol, nebivolol, metoprolol, carvedilol, betaxolol, propranolol, sotalol and atenolol), or in control participants (p = 0.27, p = 0.14, p = 0.60, p = 0.74, p = 0.98, p = 0.99, p = 0.32 for bisoprolol, nebivolol, metoprolol, carvedilol, betaxolol, sotalol and atenolol) in the pooled analysis. Similarly, no associations were found when the cohorts were analysed separately (Table S1). The further associations between the somnolent phenotype and any types of beta-blockers were not significant in those OSA patients who did not take statins and those who had polysomnography (Please see Table S1).
Bivariate analysis between ESS and clinical factors in patients with OSA and controls
In patients with OSA, a significant correlation between ESS and BMI (ρ = 0.25), TST (ρ = 0.07), AHI (ρ = 0.32), ODI (ρ = 0.33) and MinSatO2 (ρ = – 0.32, all p < 0.05) was observed. In addition, ESS was higher in patients with hypertension (10 /6–14/ vs. 8 /5–12/), diabetes (11 /7–16/ vs. 9 /5–13/) and cerebro/cardiovascular disease (11 /6–14 vs. 9 /6–13/) and in those were on statin treatment (9 /6–14/ vs 8 /5–13/, all p < 0.05). There was no relationship between ESS and age, gender or the presence of cardiac arrhythmias in the pooled analysis (all p > 0.05). In the Greek cohort, ESS was related to BMI (ρ = 0.22), TST (ρ = 0.14), AHI (ρ = 0.26), ODI (ρ = 0.25) and MinSatO2 (ρ = – 0.24, all p < 0.01). It was higher in patients with hypertension (10 /6–14/ vs. 9 /5–13/, p < 0.01). In the Hungarian cohort, ESS was related to age (ρ = – 0.13), AHI (ρ = 0.14) and ODI (ρ = 0.14). In the Moldavian cohort, ESS was related to age (ρ = 0.11), BMI (ρ = 0.39), AHI (ρ = 0.61), ODI (ρ = 0.63) and MinSatO2 (ρ = – 0.56). The ESS was higher in patients with hypertension (12 /10–15/ vs. 9 /6–11/, p < 0.01), diabetes (13 /11–17/ vs. 11 /8–14/, p < 0.01) and cerebro/cardiovascular disease (12 /10–15/ vs. 9 /6–11/, p < 0.01). None of the other correlations were significant (p > 0.05, Table S1).
In controls, ESS was only correlated to BMI (ρ = 0.14, p < 0.05) in the pooled analysis, but only in the Greek cohort (ρ = 0.16, p = 0.02) when analysing the cohorts separately.
The association between ESS and beta-blockers use in patients with OSA and controls
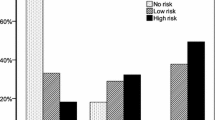
There was no difference in ESS between the patients with OSA who were using beta-blockers and non-users in the whole cohort (10 /6–14/ vs. 9 /6–13/, unadjusted p = 0.15, adjusted p = 0.19, Fig. 1a), or in the Greek cohort (p = 0.24). When the Hungarian cohort was analysed separately, patients with OSA who used beta-blockers had lower ESS (4 /3–7 vs. 6 /4–9/, p = 0.02). In contrast, in Moldavian patients, beta-blocker usage was related to higher ESS (12 /10–16/ vs. 11 /8–14/, p < 0.01).
Epworth Sleepiness Scale in patients with OSA according to the usage of beta-blockers (a) and bisoprolol (b). There was no difference between patients with OSA who took beta-blockers (BB) compared to those who did not (p = 0.15). However, patients with OSA who took bisoprolol had higher Epworth Sleepiness Scale compared to those who did not take beta-blockers (p < 0.01). Data are presented as median and interquartile range
Analysis of specific beta-blockers revealed that patients on bisoprolol had increased ESS (11 /7–15/, adjusted p < 0.01, Fig. 1b) in the pooled analysis and in the Moldavian cohort too (13 /11–16/, adjusted p < 0.01). Interestingly, in the Hungarian cohort, patients on bisoprolol had significantly lower ESS (4 /3–7/, p = 0.03). None of the other beta-blockers were significantly associated with altered ESS neither in the pooled analysis (p = 0.42, p = 0.84, p = 0.39, p = 0.40, p = 0.39, p = 0.24, p = 0.74 for nebivolol, metoprolol, carvedilol, betaxolol, propranolol, sotalol and atenolol, respectively), nor in the three cohorts separately (Please see the Table S1).
When patients taking statins were excluded from the analyses, no relationship was observed between beta-blocker use and ESS (p = 0.47). The association between ESS and bisoprolol remained significant (p < 0.01) whereas the usage of other beta-blockers was not correlated to the ESS (p > 0.05).
Analysing 1334 patients who had polysomnography, ESS did not relate to beta-blocker use (p = 0.44). Interestingly, in these patients the association between bisoprolol and ESS disappeared (p = 0.64). Of note, only 55 of the original 98 bisoprolol users had polysomnography. There was no relationship between ESS and other beta-blockers either (p > 0.05).
In the control group, there was no difference in ESS between the beta-blocker users (7 /4–10/) and non-users (7 /4–11/, unadjusted p = 0.71, adjusted p = 0.70, Fig. 2). Due to the very small number of subjects in the subgroups no further analysis on specific beta-blockers was performed.
The association between AHI and beta-blockers use in patients with OSA
Increased AHI was not related to beta-blocker (p = 0.24), bisoprolol (p = 0.29), nebivolol (p = 0.46), metoprolol (p = 0.53), carvedilol (p = 0.06), betaxolol (p = 0.50), propranolol (p = 0.62), sotalol (p = 0.79) or atenolol use (p = 0.87) in the pooled analysis. In the separate cohort analysis, we detected a significant association between AHI and carvedilol usage only in the Hungarian cohort (with carvedilol 45.3 /30.9–62.5/ vs. without carvedilol 23.9 /12.2–40.4/ p = 0.02). The further associations between AHI and any types of beta-blockers were not significant in the cohorts (Please see the Table S1).
Discussion
In a large, international sample of patients with OSA and snoring controls we demonstrated that beta-blocker use is not associated with increased daytime sleepiness.
It is known that noradrenaline is an important neurotransmitter for the maintenance of alertness [11] primarily via alpha-adrenergic receptors [12]. In contrast, the function of beta-receptors in the regulation of sleep and wakefulness has been less studied [12], but it seems they have a particular influence on rapid eye movement (REM) sleep [11]. In line with this, changes in sleep continuity and increased number of REM periods and total REM time were observed after beta-blocker therapy compared to placebo [13]. The effect of beta-blockers on REM sleep highlights the possibility that the potential association between beta-blocker use and sleepiness may not necessarily be due to provoking microarousals but disturbing the physiological sleep pattern in REM sleep.
The adrenergic system is involved in the maintenance of the patency of the upper airways [14]. Theoretically, their blockage could lead to increased collapsibility resulting higher AHI. In line with this, alpha-1 adrenergic antagonists use was associated with increased AHI in a population-based study [15]. However, in our study we did not find a significant relationship between the AHI and beta-blocker use, the only exception was carvedilol in the Hungarian subgroup.
Beta-blockers may also influence daytime sleepiness via their direct effect on the cardiovascular system. In patients with OSA increased heart-rate variability [16] and the degree of sympathetic activity [17] were associated with lower ESS. This suggests that tighter control of the heart rate by beta-blockers may paradoxically contribute to increased sleepiness. However, in our study, we did not record heart-rate variability or other markers of sympathetic activity.
Although bisoprolol was not associated with the somnolent phenotype, higher ESS values were observed in those patients who took bisoprolol. Bisoprolol is a moderately lipophilic drug that penetrates the brain, and its concentrations in the cerebrospinal fluid are comparable to those in plasma [5]. In contrast to other beta-blockers such as carvedilol or labetalol, it may also influence the production of melatonin which is a key regulator hormone of human sleep and circadian rhythm [18,19,20]. In line with this, previous studies reported a high prevalence of sleep disturbances during bisoprolol treatment [21, 22]. Bisoprolol is a highly selective beta-1-receptor antagonist. As discussed above, bisoprolol could have led to increased sleepiness due to its tighter control of heart rate [16]. It must be emphasised that the difference in ESS between bisoprolol users and those who did not take any beta-blocker was lower than the minimal clinically important difference of the ESS [23]. The effect of bisoprolol was not seen when patients who had polygraphy as a diagnostic test were excluded. Polysomnography is a gold standard in diagnosing OSA and can reveal other pathologies compared to polygraphy and it is possible that other sleep disorders were unexpectedly undiagnosed in those who had polygraphy. More importantly, nearly half of the bisoprolol users had polygraphy, therefore the lack of differences could be due to the low number of the remaining subjects. Of note, bisoprolol was the most frequently used beta-blocker in our study. Therefore, comparisons to other medications, especially their lack of association with ESS must be interpreted with caution.
There were significant differences in the demographics, prevalence of comorbidities, medication usage and severity of OSA between the three cohorts. We included patients who were originally referred with suspected OSA due to symptoms and/or phenotype suggestive for OSA, including comorbidities. Apart from a well-known geographical variability of the prevalence of smoking, obesity and cardiometabolic disease across Europe [24], marked heterogeneity of OSA severity has been reported in the European Sleep Apnoea Database [25]. To analyse if the observed heterogeneity influences our findings, we investigated the three cohorts separately. The outcomes of the primary analysis were not different in the specific cohorts compared to the pooled analysis. However, some differences were observed for secondary analyses. Of note, these analyses need to be interpreted carefully, as they have been performed in a limited number of subjects. We believe that the heterogeneity of our population apart from the large number is the strength of our manuscript.
Our study has limitations. Most importantly, the data were analysed retrospectively in a cross-sectional design. To confirm that beta-blockers do not worsen sleepiness in OSA, prospective studies are warranted. We used the ESS to evaluate sleepiness [7] which is a subjective reflection from the patients. Due to driving licence regulations, patients may underreport their actual symptoms. In addition, some patients with long-standing, slowly progressing OSA may underestimate their sleepiness. Furthermore, other subjects with chronic mental or somatic disease may report higher ESS which may reflect chronic fatigue rather than sleepiness in their case [26]. Objective tests such as the multiple sleep latency test or the maintenance of wakefulness test [27] or analysing the sleep architecture can more objectively describe sleepiness. However, the multiple sleep latency test and the maintenance of wakefulness test are not routinely used to evaluate OSA and these results were not available for us. Polysomnography is more sensitive than polygraphy and may reveal other pathologies than OSA. Our sensitivity analyses revealed that analysing only those cases where polysomnography was performed, beta-blocker use was similarly not related to ESS. We excluded patients who were taking any sedative medication. This exclusion was based on patient report and available drug charts. It is possible that the medication list was inaccurate in some patients. Although patients who took antidepressants were excluded, undiagnosed depression could have biased our data. We analysed patients who were taking statins separately. Although statins do not significantly influence sleep architecture [28] they can cause fatigue [29], which can be sometimes difficult to be differentiated from sleepiness. In line with this, higher ESS was associated with statin use in our study. However, the outcomes did not change after excluding patients on statin therapy. Finally, alcohol intake was not recorded as potential confounding factor.
In summary, beta-blocker use was not associated with increased daytime sleepiness in OSA. However, bisoprolol treatment was related to a slightly, but significantly higher ESS which warrants further investigations. Our data suggest that co-existing OSA should not discourage clinicians to prescribe beta-blocker therapy if clinically indicated. However, the development or unexplained sleepiness in patients taking these medications, in particular bisoprolol, may warrant a pausing of the beta-blocker.
References
Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22(2):6. https://doi.org/10.1007/s11886-020-1257-y.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Koella WP. CNS-related (side-)effects of beta-blockers with special reference to mechanisms of action. Eur J Clin Pharmacol. 1985;28(Suppl):55–63. https://doi.org/10.1007/bf00543711.
McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46(2):163–97. https://doi.org/10.1016/0163-7258(90)90092-g.
Sigaroudi A, Kinzig M, Wahl O, Stelzer C, Schroeter M, Fuhr U, et al. Quantification of bisoprolol and metoprolol in simultaneous human serum and cerebrospinal fluid samples. Pharmacology. 2018;101(1–2):29–34. https://doi.org/10.1159/000480091.
Poirier L, Tobe SW. Contemporary use of beta-blockers: clinical relevance of subclassification. Can J Cardiol. 2014;30(5 Suppl):S9-s15. https://doi.org/10.1016/j.cjca.2013.12.001.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. https://doi.org/10.1093/sleep/14.6.540.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. https://doi.org/10.1093/aje/kws342.
Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. https://doi.org/10.1093/sleep/28.4.499.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med JCSM. 2012;8(5):597–619. https://doi.org/10.5664/jcsm.2172.
Berridge CW, Schmeichel BE, España RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16(2):187–97. https://doi.org/10.1016/j.smrv.2011.12.003.
Broese M, Riemann D, Hein L, Nissen C. alpha-Adrenergic receptor function, arousal and sleep: mechanisms and therapeutic implications. Pharmacopsychiatry. 2012;45(6):209–16. https://doi.org/10.1055/s-0031-1299728.
Kostis JB, Rosen RC. Central nervous system effects of beta-adrenergic-blocking drugs: the role of ancillary properties. Circulation. 1987;75(1):204–12. https://doi.org/10.1161/01.cir.75.1.204.
Taranto-Montemurro L, Messineo L, Wellman A. Targeting endotypic traits with medications for the pharmacological treatment of obstructive sleep apnea. A review of the current literature. J Clin Med. 2019. https://doi.org/10.3390/jcm8111846.
Su PL, Lin WK, Lin CY, Lin SH. Alpha-1 adrenergic-antagonist use increases the risk of sleep apnea: a nationwide population-based cohort study. J Clin Sleep Med. 2019;15(11):1571–9. https://doi.org/10.5664/jcsm.8014.
Taranto Montemurro L, Floras JS, Picton P, Kasai T, Alshaer H, Gabriel JM, et al. Relationship of heart rate variability to sleepiness in patients with obstructive sleep apnea with and without heart failure. J Clin Sleep Med. 2014;10(3):271–6. https://doi.org/10.5664/jcsm.3526.
Taranto Montemurro L, Floras JS, Millar PJ, Kasai T, Gabriel JM, Spaak J, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142(5):1222–8. https://doi.org/10.1378/chest.11-2963.
Cowen PJ, Bevan JS, Gosden B, Elliott SA. Treatment with beta-adrenoceptor blockers reduces plasma melatonin concentration. Br J Clin Pharmacol. 1985;19(2):258–60. https://doi.org/10.1111/j.1365-2125.1985.tb02640.x.
Stoschitzky K, Koshucharova G, Lercher P, Maier R, Sakotnik A, Klein W, et al. Stereoselective effects of (R)- and (S)-carvedilol in humans. Chirality. 2001;13(7):342–6. https://doi.org/10.1002/chir.1042.
Stoschitzky K, Stoschitzky G, Brussee H, Bonelli C, Dobnig H. Comparing beta-blocking effects of bisoprolol, carvedilol and nebivolol. Cardiology. 2006;106(4):199–206. https://doi.org/10.1159/000093060.
Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005;144(3):317–22. https://doi.org/10.1038/sj.bjp.0706048.
Kohli RS, Khurmi NS, Kardash MM, Hughes LO, Lahiri A, Raftery EB. Efficacy of once daily bisoprolol in stable angina pectoris: an objective comparison with atenolol and long term follow-up. Eur Heart J. 1985;6(10):845–50. https://doi.org/10.1093/oxfordjournals.eurheartj.a061771.
Patel S, Kon SSC, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The epworth sleepiness scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–3. https://doi.org/10.1164/rccm.201704-0672LE.
OECDLibrary. Health at a Glance. 2019. https://www.oecd-ilibrary.org.
Hedner J, Grote L, Bonsignore M, McNicholas W, Lavie P, Parati G, et al. The european sleep apnoea database (ESADA): report from 22 European sleep laboratories. Eur Respir J. 2011;38(3):635–42. https://doi.org/10.1183/09031936.00046710.
Chervin RD. The multiple sleep latency test and Epworth sleepiness scale in the assessment of daytime sleepiness. J Sleep Res. 2000;9(4):399–401. https://doi.org/10.1046/j.1365-2869.2000.0227a.x.
Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9(1):5–11. https://doi.org/10.1046/j.1365-2869.2000.00177.x.
Broncel M, Gorzelak-Pabis P, Sahebkar A, Serejko K, Ursoniu S, Rysz J, et al. Sleep changes following statin therapy: a systematic review and meta-analysis of randomized placebo-controlled polysomnographic trials. Arch Med Sci. 2015;11(5):915–26. https://doi.org/10.5114/aoms.2015.54841.
Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–2. https://doi.org/10.1001/archinternmed.2012.2171.
Funding
Open access funding provided by Semmelweis University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. AM, JV and AB were supported by the NIHR Manchester BRC. Financial/nonfinancial disclosures: None reported.
Author information
Authors and Affiliations
Contributions
AB, AC and PS conceived the study and participated in study design. AB, MM, AC and PS performed sleep studies and analysed data. AB and MM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The manuscript was drafted by MM and AB and was critically reviewed and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any conflict of interest.
Ethical committee permission
The studies have been approved by the local ethics committees (Semmelweis University TUKEB 30/2014, RKEB 172/2018, University General Hospital of Alexandroupolis 29/SB5/2014, State University of Medicine and Pharmacy "Nicolae Testemitanu" 39/44).
Informed consent
All participants gave their written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meszaros, M., Mathioudakis, A.G., Xanthoudaki, M. et al. The association between beta-blocker therapy and daytime sleepiness in obstructive sleep apnoea. Sleep Biol. Rhythms 19, 399–408 (2021). https://doi.org/10.1007/s41105-021-00330-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-021-00330-z