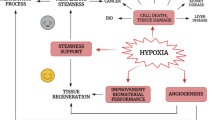
Abstract
Clinically, timely reperfusion strategies to re-establish oxygenated blood flow in ischemic heart diseases seem to salvage viable myocardium effectively. Despite the remarkable improvement in cardiac function, reperfusion therapy could paradoxically trigger hypoxic cellular injury and dysfunction. Experimental laboratory models have been developed over the years to explain better the pathophysiology of cardiac ischemia–reperfusion injury, including the in vitro hypoxia-reoxygenation cardiac injury model. Furthermore, the use of nutritional myocardial conditioning techniques have been successful. The cardioprotective potential of flavonoids have been greatly linked to its anti-oxidant, anti-apoptotic and anti-inflammatory properties. While several studies have reviewed the cardioprotective properties of flavonoids, there is a scarce evidence of their function in the hypoxia-reoxygenation injury cell culture model. Hence, the aim of this review was to lay out and summarize our current understanding of flavonoids’ function in mitigating hypoxia-reoxygenation cardiac injury based on evidence from the last five years. We also discussed the possible mechanisms of flavonoids in modulating the cardioprotective effects as such information would provide invaluable insight on future therapeutic application of flavonoids.



Similar content being viewed by others
References
World Health Organization. (2021). Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases
Ministry of Health Malaysia. (2020). The impact of noncommunicable diseases and their risk factors on Malaysia’s gross domestic product.
McDougal, A. D., & Dewey, C. F., Jr. (2017). Modeling oxygen requirements in ischemic cardiomyocytes. Journal of Biological Chemistry, 292(28), 11760–11776. https://doi.org/10.1074/jbc.M116.751826
Severino, P., D’Amato, A., Pucci, M., Infusino, F., Adamo, F., Birtolo, L. I., Netti, L., Montefusco, G., Chimenti, C., Lavalle, C., & Maestrini, V. (2020). Ischemic heart disease pathophysiology paradigms overview: From plaque activation to microvascular dysfunction. International Journal of Molecular Sciences, 21(21), 8118. https://doi.org/10.3390/ijms21218118
Katz, D., & Gavin, M. C. (2019). Stable ischemic heart disease. Annals of Internal Medicine, 171(3), 8060.
Wu, M. Y., Yiang, G. T., Liao, W. T., Tsai, A. P. Y., Cheng, Y. L., Cheng, P. W., Li, C. Y., & Li, C. J. (2018). Current mechanistic concepts in ischemia and reperfusion injury. Cellular Physiology and Biochemistry, 46(4), 1650–1667. https://doi.org/10.1159/000489241
Cowled, P., & Fitridge, R. (2011). Mechanisms of vascular disease: Pathophysiology of reperfusion injury. Adelaide (AU): University of Adelaide Press.
Neri, M., Riezzo, I., Pascale, N., Pomara, C., & Turillazzi, E. (2017). Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediators of Inflammation, 2017, 7018393. https://doi.org/10.1155/2017/7018393
Chakraborti, S., Dhalla, N. S., Dikshit, M., & Ganguly, N. K. (2019). Modulation of oxidative stress in heart disease. Singapore: Springer.
Lindsey, M. L., Bolli, R., Canty, J. M., Jr., Du, X. J., Frangogiannis, N. G., Frantz, S., Gourdie, R. G., Holmes, J. W., Jones, S. P., Kloner, R. A., & Lefer, D. J. (2018). Guidelines for experimental models of myocardial ischemia and infarction. American Journal of Physiology, 314(4), H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Donato, M., Evelson, P., & Gelpi, R. J. (2017). Protecting the heart from ischemia/reperfusion injury: An update on remote ischemic preconditioning and postconditioning. Current Opinion in Cardiology, 32(6), 784–790. https://doi.org/10.1097/HCO.0000000000000447
Da Zhou, J. D., Ya, J., Pan, L., Wang, Y., Ji, X., & Meng, R. (2018). Remote ischemic conditioning: A promising therapeutic intervention for multi-organ protection. Aging, 10(8), 1825. https://doi.org/10.18632/aging.101527
Jiang, H., Xing, J., Fang, J., Wang, L., Wang, Y., Zeng, L., Li, Z., & Liu, R. (2020). Tilianin Protects against ischemia/reperfusion-induced myocardial injury through the inhibition of the Ca2+/calmodulin-dependent protein kinase II-dependent apoptotic and inflammatory signalling pathways. BioMed Research International, 2020, 5939715. https://doi.org/10.1155/2020/5939715
Liu, G., Zhang, B. F., Hu, Q., Liu, X. P., & Chen, J. (2020). Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signalling pathway. Biochemical and Biophysical Research Communications, 531(2), 242–249. https://doi.org/10.1016/j.bbrc.2020.07.047
Yao, X., Jiao, S., Qin, M., Hu, W., Yi, B., & Liu, D. (2020). Vanillic acid alleviates acute myocardial hypoxia/reoxygenation injury by inhibiting oxidative stress. Oxidative Medicine and. Cellular Longevity, 2020, 8348035. https://doi.org/10.1155/2020/8348035
Wang, M., Liu, Y., Pan, R. L., Wang, R. Y., Ding, S. L., Dong, W. R., Sun, G. B., Ye, J. X., & Sun, X. B. (2019). Protective effects of Myrica rubra flavonoids against hypoxia/reoxygenation-induced cardiomyocyte injury via the regulation of the PI3K/Akt/GSK3β pathway. International Journal of Molecular Medicine, 43(5), 2133–2143. https://doi.org/10.3892/ijmm.2019.4131
Yang, P., Zhou, Y., Xia, Q., Yao, L., & Chang, X. (2019). Astragaloside IV regulates the PI3K/Akt/HO-1 signalling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia–reoxygenation. Biological and Pharmaceutical Bulletin, 42(5), 721–727. https://doi.org/10.1248/bpb.b18-00854
Wu, W. Y., Li, Y. D., Cui, Y. K., Wu, C., Hong, Y. X., Li, G., Wu, Y., Jie, L. J., Wang, Y., & Li, G. R. (2018). The natural flavone acacetin confers cardiomyocyte protection against hypoxia/reoxygenation injury via AMPK-mediated activation of Nrf2 signalling pathway. Frontiers in Pharmacology, 9, 497. https://doi.org/10.3389/fphar.2018.00497
Zhang, H. J., Chen, R. C., Sun, G. B., Yang, L. P., Xu, X. D., & Sun, X. B. (2018). Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine, 40, 88–97. https://doi.org/10.1016/j.phymed.2018.01.004
Ali, S. S., Ahmad, W. A. N. W., Budin, S. B., & Zainalabidin, S. (2020). Implication of dietary phenolic acids on inflammation in cardiovascular disease. Reviews in Cardiovascular Medicine, 21(2), 225–240. https://doi.org/10.31083/j.rcm.2020.02.49
Mulvihill, E. E., Burke, A. C., & Huff, M. W. (2016). Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annual Review of Nutrition, 36, 275–299. https://doi.org/10.1146/annurev-nutr-071715-050718
Marunaka, Y., Marunaka, R., Sun, H., Yamamoto, T., Kanamura, N., Inui, T., & Taruno, A. (2017). Actions of quercetin, a polyphenol, on blood pressure. Molecules, 22(2), 209. https://doi.org/10.3390/molecules22020209
Eng, Q. Y., Thanikachalam, P. V., & Ramamurthy, S. (2018). Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. Journal of Ethnopharmacology, 210, 296–310. https://doi.org/10.1016/j.jep.2017.08.035
Choy, K. W., Murugan, D., Leong, X. F., Abas, R., Alias, A., & Mustafa, M. R. (2019). Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signalling in cardiovascular diseases: A mini review. Frontiers in Pharmacology, 10, 1295. https://doi.org/10.3389/fphar.2019.01295
Ciumărnean, L., Milaciu, M. V., Runcan, O., Vesa, ȘC., Răchișan, A. L., Negrean, V., Perné, M. G., Donca, V. I., Alexescu, T. G., Para, I., & Dogaru, G. (2020). The effects of flavonoids in cardiovascular diseases. Molecules, 25(18), 4320. https://doi.org/10.3390/molecules25184320
Rufino, A. T., Costa, V. M., Carvalho, F., & Fernandes, E. (2021). Flavonoids as antiobesity agents: A review. Medicinal Research Reviews, 41(1), 556–585. https://doi.org/10.1002/med.21740
Son, E., Lee, D., Woo, C. W., & Kim, Y. H. (2020). The optimal model of reperfusion injury in vitro using H9c2 transformed cardiac myoblasts. Korean Journal of Physiology Pharmacology, 24(2), 173. https://doi.org/10.4196/kjpp.2020.24.2.173
Gerő, D. (2017). Hypoxia and human diseases: The hypoxia-reoxygenation injury model. Intech Open, 47, 65339.
Kuznetsov, A. V., Javadov, S., Sickinger, S., Frotschnig, S., & Grimm, M. (2015). H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochimica et Biophysica Acta, Molecular Cell Research, 1853(2), 276–284. https://doi.org/10.1016/j.bbamcr.2014.11.015
Pavlacky, J., & Polak, J. (2020). Technical feasibility and physiological relevance of hypoxic cell culture models. Frontiers in Endocrinology, 11, 57. https://doi.org/10.3389/fendo.2020.00057
Sayed, N., Tambe, P., Kumar, P., Jadhav, S., Paknikar, K. M., & Gajbhiye, V. (2020). miRNA transfection via poly (amidoamine)-based delivery vector prevents hypoxia/reperfusion-induced cardiomyocyte apoptosis. Nanomedicine, 15(2), 163–181. https://doi.org/10.2217/nnm-2019-0363
Teti, G., Focaroli, S., Salvatore, V., Mazzotti, E., Ingra, L., Mazzotti, A., & Falconi, M. (2018). (2018) The hypoxia-mimetic agent cobalt chloride differently affects human mesenchymal stem cells in their chondrogenic potential. Stem Cells International, 2018, 3237253. https://doi.org/10.1155/2018/3237253
Jennings, R. Á. (1960). Myocardial necrosis induced by temporary occulusion of a coronary artery in the dog. Archives of Pathology, 70, 68–70.
Douzinas, E. E., & Apeiranthitis, A. (2019). Modulation of oxidative stress in heart disease: basic mechanisms of ischemia/reperfusion injury leading to cellular and tissue damage: Therapeutic implications. Singapore: Springer.
Eltzschig, H. K., & Eckle, T. (2011). Ischemia and reperfusion—from mechanism to translation. Nature Medicine, 17(11), 1391–1401. https://doi.org/10.1038/nm.2507
Frank, A., Bonney, M., Bonney, S., Weitzel, L., Koeppen, M., & Eckle, T. (2012) Myocardial ischemia-reperfusion injury: From basic science to clinical bedside. Seminars in Cardiothoracic and Vascular Anesthesia, 16(3), 123–132. https://doi.org/10.1177/1089253211436350
Yang, C. F. (2018). Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Tzu-Chi Med. J., 30(4), 209. https://doi.org/10.4103/tcmj.tcmj_33_18
Kurian, G. A., Rajagopal, R., Vedantham, S., & Rajesh, M. (2016). The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxidative Medicine and Cellular Longevity, 2016, 1656450. https://doi.org/10.1155/2016/1656450
Jubaidi, F. F., Zainalabidin, S., Mariappan, V., & Budin, S. B. (2020). Mitochondrial dysfunction in diabetic cardiomyopathy: The possible therapeutic roles of phenolic acids. International Journal of Molecular Sciences, 21(17), 6043. https://doi.org/10.3390/ijms21176043
Dias, A. E. M. S. Á. S., Melnikov, P., & Cônsolo, L. Z. Z. (2015). Oxidative stress in coronary artery bypass surgery. Brazilian Journal of Cardiovascular Surgery, 30(4), 417–424. https://doi.org/10.5935/1678-9741.20150052
Liu, J., Hou, J., Xia, Z. Y., Zeng, W., Wang, X., Li, R., Ke, C., Xu, J., Lei, S., & Xia, Z. (2013). Recombinant PTD-Cu/Zn SOD attenuates hypoxia–reoxygenation injury in cardiomyocytes. Free Rad. Res., 47(5), 386–393. https://doi.org/10.3109/10715762.2013.780286
Pei, H., Yang, Y., Zhao, H., Li, X., Yang, D., Li, D., & Yang, Y. (2016). The role of mitochondrial functional proteins in ROS production in ischemic heart diseases. Oxidative Medicine and Cellular Longevity, 2016, 5470457. https://doi.org/10.1155/2016/5470457
Hausenloy, D. J., & Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. The Journal of Clinical Investigation, 123(1), 92–100. https://doi.org/10.1172/JCI62874
Barkhade, T., Mahapatra, S. K., & Banerjee, I. (2019). Study of mitochondrial swelling, membrane fluidity and ROS production induced by nano-TiO2 and prevented by Fe incorporation. Toxicology Research, 8(5), 711–722. https://doi.org/10.1039/C9TX00143C
Zhang, C. X., Cheng, Y., Liu, D. Z., Liu, M., Cui, H., Mei, Q. B., & Zhou, S. Y. (2019). Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. Journal of Nanobiotechnology, 17(1), 1–16. https://doi.org/10.1186/s12951-019-0451-9
Gendron, A., Lan, L., Tran, N., Laloy, J., Brusini, R., Rachet, A., Gobeaux, F., Nicolas, V., Chaminade, P., Abreu, S., Desmaële, D., & Varna, M. (2021). New nanoparticle formulation for cyclosporin A: In vitro assessment. Pharmaceutics, 13(1), 91. https://doi.org/10.3390/pharmaceutics13010091
González-Montero, J., Brito, R., Gajardo, A. I., & Rodrigo, R. (2018). Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World Journal of Cardiology, 10(9), 74. https://doi.org/10.4330/wjc.v10.i9.74
He, X., Li, S., Fang, X., & Liao, Y. (2018). TDCPP protects cardiomyocytes from hypoxia-reoxygenation injury induced apoptosis through mitigating calcium overload and promotion GSK-3β phosphorylation. Regulatory Toxicology and Pharmacology, 92, 39–45. https://doi.org/10.1016/j.yrtph.2017.11.005
Landstrom, A. P., Dobrev, D., & Wehrens, X. H. (2017). Calcium signalling and cardiac arrhythmias. Circulation Research, 120(12), 1969–1993. https://doi.org/10.1161/CIRCRESAHA.117.310083
Pittas, K., Vrachatis, D. A., Angelidis, C., Tsoucala, S., Giannopoulos, G., & Deftereos, S. (2018). The role of calcium handling mechanisms in reperfusion injury. Current Pharmaceutical Design, 24(34), 4077–4089. https://doi.org/10.2174/1381612825666181120155953
Garcia-Dorado, D., Ruiz-Meana, M., Inserte, J., Rodriguez-Sinovas, A., & Piper, H. M. (2012). Calcium-mediated cell death during myocardial reperfusion. Cardiovascular Research, 94(2), 168–180. https://doi.org/10.1093/cvr/cvs116
Kalogeris, T., Baines, C. P., Krenz, M., & Korthuis, R. J. (2012). Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology, 298, 229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Minato, H., Hisatome, I., Kurata, Y., Notsu, T., Nakasone, N., Ninomiya, H., Hamada, T., Tomomori, T., Okamura, A., Miake, J., & Tsuneto, M. (2020). Pretreatment with cilnidipine attenuates hypoxia/reoxygenation injury in HL-1 cardiomyocytes through enhanced NO production and action potential shortening. Hyperten. Res., 43(5), 380–388. https://doi.org/10.1038/s41440-019-0391-7
Mahmoud, A. M., Hernandez Bautista, R. J., Sandhu, M. A., & Hussein, O. E. (2019). Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Medicine and Cellular Longevity, 2019, 548413. https://doi.org/10.1155/2019/5484138
Xiao, J. (2017). Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Critical Reviews in Food Science and Nutrition, 57(9), 1874–1905. https://doi.org/10.1080/10408398.2015.1032400
Abbas, M., Saeed, F., Anjum, F. M., Afzaal, M., Tufail, T., Bashir, M. S., Ishtiaq, A., Hussain, S., & Suleria, H. A. R. (2017). Natural polyphenols: An overview. International Journal of Food Properties, 20(8), 1689–1699. https://doi.org/10.1080/10942912.2016.1220393
Crozier, A., Del Rio, D., & Clifford, M. N. (2010). Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine, 31(6), 446–467. https://doi.org/10.1016/j.mam.2010.09.007
Murota, K., Nakamura, Y., & Uehara, M. (2018). Flavonoid metabolism: The interaction of metabolites and gut microbiota. Bioscience Biotechnology Biochemistry, 82(4), 600–610. https://doi.org/10.1080/09168451.2018.1444467
Zhao, J., Yang, J., & Xie, Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. International Journal of Pharmaceutics, 570, 118642. https://doi.org/10.1016/j.ijpharm.2019.118642
Hostetler, G. L., Ralston, R. A., & Schwartz, S. J. (2017). Flavones: Food sources, bioavailability, metabolism, and bioactivity. Advances in Nutrition, 8(3), 423–435. https://doi.org/10.3945/an.116.012948
Qiao, Z., Xu, Y. W., & Yang, J. (2016). Eupatilin inhibits the apoptosis in H9c2 cardiomyocytes via the Akt/GSK-3β pathway following hypoxia/reoxygenation injury. Biomedicine Pharmatherapy, 82, 373–378. https://doi.org/10.1016/j.biopha.2016.05.026
Gallyas, F., Jr., Sumegi, B., & Szabo, C. (2020). Role of Akt activation in PARP inhibitor resistance in cancer. Cancers, 12(3), 532. https://doi.org/10.3390/cancers12030532
Tang, J. Y., Jin, P., He, Q., Lu, L. H., Ma, J. P., Gao, W. L., Bai, H. P., & Yang, J. (2017). Naringenin ameliorates hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated apoptosis in H9c2 myocardial cells: Involvement in ATF6, IRE1α and PERK signalling activation. Molecular and Cellular Biochemistry, 424(1–2), 111–122. https://doi.org/10.1007/s11010-016-2848-1
Liu, L., Wu, Y., & Huang, X. (2016). Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy. European Journal of Pharmacology, 776, 90–98. https://doi.org/10.1016/j.ejphar.2016.02.037
Wu, W. Y., Li, Y. D., Cui, Y. K., Wu, C., Hong, Y. X., Li, G., Wu, Y., Jie, L. J., Wang, Y., & Li, G. R. (2019). The natural flavone acacetin confers cardiomyocyte protection against hypoxia/reoxygenation injury via AMPK-mediated activation of Nrf2 signalling pathway. Frontiers in Pharmacology, 9, 497. https://doi.org/10.3389/fphar.2018.00497
Pang, J. J., Barton, L. A., Chen, Y. G., & Ren, J. (2015). Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: From bench to bedside. Acta Physcologica Sinica, 67(6), 535–544. https://doi.org/10.1007/978-981-13-6260-6_6
Jiang, W. B., Zhao, W., Chen, H., Wu, Y. Y., Wang, Y., Fu, G. S., & Yang, X. J. (2018). Baicalin protects H9c2 cardiomyocytes against hypoxia/reoxygenation-induced apoptosis and oxidative stress through activation of mitochondrial aldehyde dehydrogenase 2. Clinical and Experimental Pharmacology and Physiology, 45(3), 303–311. https://doi.org/10.1111/1440-1681.12876
Wang, Y., Wang, Y., Wang, X., & Hu, P. (2018). Tilianin-loaded reactive oxygen species-scavenging nano-micelles protect H9c2 cardiomyocyte against hypoxia/reoxygenation-induced injury. Journal of Cardiovascular Pharmacology, 72(1), 32–39. https://doi.org/10.1097/FJC.0000000000000587
Chen, S., Yang, B., Xu, Y., Rong, Y., & Qiu, Y. (2018). Protection of Luteolin-7-O-glucoside against apoptosis induced by hypoxia/reoxygenation through the MAPK pathways in H9c2 cells. Molecular Medicine Reports, 17(5), 7156–7162. https://doi.org/10.3892/mmr.2018.8774
Liu, Z., Yang, L., Huang, J., Xu, P., Zhang, Z., Yin, D., Liu, J., He, H., & He, M. (2018). Luteoloside attenuates anoxia/reoxygenation-induced cardiomyocytes injury via mitochondrial pathway mediated by 14–3-3η protein. Phytotherapy Research, 32(6), 1126–1134. https://doi.org/10.1002/ptr.6053
Xue, W., Wang, X., Tang, H., Sun, F., Zhu, H., Huang, D., & Dong, L. (2020). Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomedicine & Pharmacotherapy, 124, 109849. https://doi.org/10.1016/j.biopha.2020.109849
Parra, V., Eisner, V., Chiong, M., Criollo, A., Moraga, F., Garcia, A., Härtel, S., Jaimovich, E., Zorzano, A., Hidalgo, C., & Lavandero, S. (2018). Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovascular Research, 77(2), 387–397. https://doi.org/10.1093/cvr/cvm029
Herrero, M., Plaza, M., Cifuentes, A., & Ibáñez, E. (2012). Extraction techniques for the determination of phenolic compounds in food. Intech Open. https://doi.org/10.5772/intechopen.84705
Huang, L., He, H., Liu, Z., Liu, D., Yin, D., & He, M. (2016). Protective effects of isorhamnetin on cardiomyocytes against anoxia/reoxygenation-induced injury is mediated by SIRT1. Journal of Cardiovascular Pharmacology, 67(6), 526–537. https://doi.org/10.1097/FJC.0000000000000376
Zhao, T. T., Yang, T. L., Gong, L., & Wu, P. (2018). Isorhamnetin protects against hypoxia/reoxygenation-induced injure by attenuating apoptosis and oxidative stress in H9c2 cardiomyocytes. Gene, 666, 92–99. https://doi.org/10.1016/j.gene.2018.05.009
Wang, M., Sun, G. B., Du, Y. Y., Tian, Y., Liao, P., Liu, X. S., Ye, J. X., & Sun, X. B. (2017). Myricitrin protects cardiomyocytes from hypoxia/reoxygenation injury: Involvement of heat shock protein 90. Frontiers in Pharmacology, 8, 353. https://doi.org/10.3389/fphar.2017.00353
Xiao, R., Xiang, A. L., Pang, H. B., & Liu, K. Q. (2017). Hyperoside protects against hypoxia/reoxygenation induced injury in cardiomyocytes by suppressing the Bnip3 expression. Gene, 629, 86–91. https://doi.org/10.1016/j.gene.2017.07.063
Cao, H., Xu, H., Zhu, G., & Liu, S. (2017). Isoquercetin ameliorated hypoxia/reoxygenation-induced H9C2 cardiomyocyte apoptosis via a mitochondrial-dependent pathway. Biomedicine & Pharmacotherapy, 95, 938–943. https://doi.org/10.1016/j.biopha.2017.08.128
Liu, S., Wu, N., Miao, J., Huang, Z., Li, X., Jia, P., Guo, Y., & Jia, D. (2018). Protective effect of morin on myocardial ischemia-reperfusion injury in rats. International Journal of Molecular Medicine, 42(3), 1379–1390. https://doi.org/10.3892/ijmm.2018.3743
Lozano, O., Lázaro-Alfaro, A., Silva-Platas, C., Oropeza-Almazán, Y., Torres-Quintanilla, A., Bernal-Ramírez, J., Alves-Figueiredo, H., & García-Rivas, G. (2019). Nanoencapsulated quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxidative Medicine and Cellular Longevity, 2019, 7683051. https://doi.org/10.1155/2019/7683051
Yang, H., Wang, C., Zhang, L., Lv, J., & Ni, H. (2019). Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial cells by upregulating SIRT1 expression. Chemico Biological Interactions, 297, 44–49. https://doi.org/10.1016/j.cbi.2018.10.016
Huang, J., & Qi, Z. (2020). MiR-21 mediates the protection of kaempferol against hypoxia/reoxygenation-induced cardiomyocyte injury via promoting Notch1/PTEN/AKT signalling pathway. PLoS ONE, 15(11), e0241007. https://doi.org/10.1371/journal.pone.0241007
Rodius, S., de Klein, N., Jeanty, C., Sánchez-Iranzo, H., Crespo, I., Ibberson, M., Xenarios, I., Dittmar, G., Mercader, N., Niclou, S. P., & Azuaje, F. (2020). Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Science and Reports, 10(1), 1–12. https://doi.org/10.1038/s41598-020-59894-4
Raman, G., Avendano, E. E., Chen, S., Wang, J., Matson, J., Gayer, B., Novotny, J. A., & Cassidy, A. (2019). Dietary intakes of flavan-3-ols and cardiometabolic health: Systematic review and meta-analysis of randomised trials and prospective cohort studies. American Journal of Clinical Nutrition, 110(5), 1067–1078. https://doi.org/10.1093/ajcn/nqz178
Liu, S., Ai, Q., Feng, K., Li, Y., & Liu, X. (2016). The cardioprotective effect of dihydromyricetin prevents ischemia–reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1α signalling pathways. Apoptosis, 21(12), 1366–1385. https://doi.org/10.1007/s10495-016-1306-6
Wu, Y., Xia, Z. Y., Zhao, B., Leng, Y., Dou, J., Meng, Q. T., Lei, S. Q., Chen, Z. Z., & Zhu, J. (2017). Epigallocatechin-3-gallate attenuates myocardial injury induced by ischemia/reperfusion in diabetic rats and in H9c2 cells under hyperglycemic conditions. International Journal of Molecular Medicine, 40(2), 389–399. https://doi.org/10.3892/ijmm.2017.3014
Zhang, C., Liao, P., Liang, R., Zheng, X., & Jian, J. (2019). Epigallocatechin gallate prevents mitochondrial impairment and cell apoptosis by regulating miR-30a/p53 axis. Phytomedicine, 61, 152845. https://doi.org/10.1016/j.phymed.2019.152845
Kozlowska, A., & Szostak-Wegierek, D. (2014). Flavonoids-food sources and health benefits. Roczniki Państwowego Zakładu Higieny, 65(2), 79.
He, S., Wang, X., Zhong, Y., Tang, L., Zhang, Y., Ling, Y., Tan, Z., Yang, P., & Chen, A. (2017). Hesperetin post-treatment prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating PI3K/Akt signalling pathway. Biomedicine & Pharmacotherapy, 91, 1106–1112. https://doi.org/10.1016/j.biopha.2017.05.003
Tang, J.-Y., Ping, J., Qing, H., Lu, L.-H., Ma, J.-P., Gao, W.-L., Bai, H.-P., & Yang, J. (2017). Naringenin ameliorates hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated apoptosis in H9c2 myocardial cells: involvement in ATF6, IRE1α and PERK signalling activation. Molecular Cellular and Biochemistry, 2017(424), 111–122.
Xie, Y., Ji, R., & Han, M. (2019). Eriodictyol protects H9c2 cardiomyocytes against the injury induced by hypoxia/reoxygenation by improving the dysfunction of mitochondria. Experimental and Therapeutic Medicine, 17(1), 551–557. https://doi.org/10.3892/etm.2018.6918
Thrane, M., Paulsen, P. V., Orcutt, M. W., & Krieger, T. M. (2017). Sustainable protein sources: Soy protein: Impacts, production, and applications. CAmbridge: Academic Press.
Ma, Y., Gai, Y., Yan, J., Jian, J., & Zhang, Y. (2016). Puerarin attenuates anoxia/reoxygenation injury through enhancing Bcl-2 associated athanogene 3 expression, a modulator of apoptosis and autophagy. International Journal of Clinical and Experimental Medicine Research, 22, 977. https://doi.org/10.12659/MSM.897379
Tang, H., Song, X., Ling, Y., Wang, X., Yang, P., Luo, T., & Chen, A. (2017). Puerarin attenuates myocardial hypoxia/reoxygenation injury by inhibiting autophagy via the Akt signalling pathway. Molecular Medicine Reports, 15(6), 3747–3754. https://doi.org/10.3892/mmr.2017.6424
Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research, 61(1), 1361779. https://doi.org/10.1080/16546628.2017.1361779
Wang, X., Jia, D., Zhang, J., & Wang, W. (2017). Grape seed proanthocyanidins protect cardiomyocytes against hypoxia/reoxygenation injury by attenuating endoplasmic reticulum stress through PERK/eIF2α pathway. Molecular Medicine Reports, 16(6), 9189–9196. https://doi.org/10.3892/mmr.2017.7756
Acknowledgements
This work was supported by the Herbal Research Grant Scheme by the Malaysian Ministry of Agriculture (Grant code304.PPSK.6150169.K123) and S.S.A received funding from GRA-ASSIST USM 2020/2021. Thank you to all of the peer reviewers and editors for their opinions and suggestions.
Funding
This work was supported by the Herbal Research Grant Scheme by the Malaysian Ministry of Agriculture (Grant code304.PPSK.6150169.K123) and S.S.A received funding from GRA-ASSIST USM 2020/2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Y. Robert Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, S.S., Noordin, L., Bakar, R.A. et al. Current Updates on Potential Role of Flavonoids in Hypoxia/Reoxygenation Cardiac Injury Model. Cardiovasc Toxicol 21, 605–618 (2021). https://doi.org/10.1007/s12012-021-09666-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09666-x