Abstract
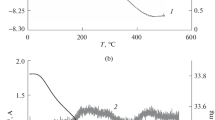
A composite aerogel with superhydrophobic external surface has been synthesized from reduced graphene oxide and polytetrafluoroethylene taken in a weight ratio of 1 : 1. The porous structure of the aerogel has been studied by the standard contact porosimetry method (SCPM). The porosimetric curves measured with respect to octane and water intersect in the region of small pores, thereby leading to the fact that the specific surface area of the aerogel in water is much larger than that in octane, although octane is known to wet any material almost ideally. This phenomenon, which is referred to as “superhydrophilicity,” is explained by the fact that, in the region of mesopores, a sample swells in water due to the hydration of surface –CO and –COH groups, which have been identified with the help of IR and Raman spectroscopies. Thus, the outside surface of the aerogel granules is superhydrophobic, while their interior is superhydrophilic in the region of small pores. As follows from the SCPM data, the total porosity and specific surface area of the aerogel are substantially larger than those of Vulcan XC-72 carbon black, which is a standard carrier for Pt catalysts used in fuel cells based on proton-exchange membranes. Oxygen electroreduction at the aerogel, containing Pt deposited in an amount of 28 µg/cm2, has been studied by the method of rotating disk electrode (RDE) in an aqueous 0.5 M H2SO4 solution, and the results obtained have been compared with the data on standard commercial Pt (20%)/Vulcan XC-72 catalyst. It has been shown that the limiting diffusion RDE currents for Pt supported on the hydrophobic–hydrophilic aerogel are markedly higher than those for the standard catalyst because of the easier access of oxygen to the reaction zone as compared with hydrophilic Vulcan XC-72 carbon black carrier.








Similar content being viewed by others
REFERENCES
Zisman, W.A., Contact angle, wettability and adhesion, in Advances in Chemistry Series, Washington, DC: Am. Chem. Soc., 1964, vol. 43, p. 513.
Volfkovich, Yu.M., Filippov, A.N., and Bagotsky, V.S., Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology, London: Springer, 2014.
Gottesfeld, S. and Zawodzinski, T.A., in Advances in Electrochemical Science and Engineering, Alkire, L.C., Gerischer, H., Kolb, D.M., and Tobias, C.W., Eds., Toronto: Wiley, 1997, p. 195.
Bagotsky, V.S., Skundin, A.M., and Volfkovich, Yu.M., Electrochemical Power Sources. Batteries, Fuel Cells, and Supercapacitors, Hoboken, New Jersey: Wiley, 2015.
Huang, Y., Liu, W., Kan, S., Liu, P., Hao, R., Hu, H., Zhang, J., Liu, H., Liu, M., and Liu, K., Int. J. Hydrogen Energy, 2020, vol. 45, p. 6380.
Sui, S., Wang, X., Zhou, X., Su, Y., Riffat, S., and Liu, C., J. Mater. Chem. A, 2017, vol. 5, p. 1808.
Zhang, J., Mo, Y., Vukmirovic, M.B., Klie, R., Sasaki, K., and Adzic, R.R., J. Phys. Chem. B, 2004, vol. 108, p. 10955.
Sharma, S. and Pollet, B.G., J. Power Sources, 2012, vol. 208, p. 96.
Görlin, M., Ferreira de Araújo, J., Schmies, H., Bernsmeier, D., Dresp, S., Gliech, M., Jusus, Z., Chernev, P., Kraehnert, R., Dau, H., and Strasser, P., J. Am. Chem. Soc., 2017, vol. 139, p. 2070.
Zhang, Y., Wang, Y., Huang, J., Han, C., and Zang, J., Int. J. Hydrogen Energy, 2020, vol. 45, p. 6529.
Zaragoza-Martín, F., Sopena-Escario, D., and Morallon, E., Salinas-Martínez de Lecea, C., J. Power Sources, 2007, vol. 171, p. 302.
Stevanovic, S.I., Panic, V.V., Dekanski, A.B., Tripkovic, A.V., and Jovanovic, V.M., Phys. Chem. Chem. Phys., 2012, vol. 14, p. 9475.
Dong, L., Gari, R.S., Li, Z., Craig, M.M., and Hou, S., Carbon, 2010, vol. 48, p. 781.
Wang, C., Li, H., Zhao, J., Zhu, Y., Yuan, W.Z., and Zhang, Y., Int. J. Hydrogen Energy, 2013, vol. 38, p. 13230.
Sosenkin, V.E., Aleksenko, A.E., Rychagov, A.Yu., Mayorova, N.A., Ovchinnikov-Lazarev, M.A., Spitsyn, B.V., and Vol’fkovich, Yu.M., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, p. 646.
Bo, X. and Guo, L., Electrochim. Acta, 2013, vol. 90, p. 283.
Chen, W., Xin, Q., Sun, G., Wang, Q., Mao, Q., and Su, H., J. Power Sources, 2008, vol. 180, p. 199.
Mikhaylova, A.A., Tusseeva, E.K., Mayorova, N.A., Rychagov, A.Yu., Volfkovich, Yu.M., Krestinin, A.V., and Khazova, O.A., Electrochim. Acta, 2011, vol. 56, p. 3656.
Calvillo, L., Celorrio, V., Moliner, R., and Lazaro, M.J., Mater. Chem. Phys., 2011, vol. 127, p. 335.
Volfkovich, Yu.M. and Sosenkin, V.E., Russ. Chem. Rev., 2012, vol. 81, p. 936.
Baskakov, S.A., Baskakova, Y.V., Kabachkov, E.N., Dremova, N.N., Michtchenko, A., and Shulga, Yu.M., ACS Appl. Mater. Interfaces, 2019, vol. 35, p. 32517.
Boinovich, L.B. and Emelyanenko, A.M., Russ. Chem. Rev., 2008, vol. 77, p. 583.
http://www.ras.ru/news/shownews.aspx?id=b53dad2c-d02c-44b3-8955-305136cb8a30.
Volfkovich, Yu.M., Sosenkin, V.E., Mayorova, N.A., Rychagov, A.Y., Baskakov, S.A., Kabachkov, E.N., Korepanov, V.I., Dremova, N.N., Baskakova, Y.V., and Shulga, Yu.M., Energy Fuels, 2020, vol. 34, p. 7573.
Bagotzky, V.S., J. Power Sources, 1994, vol. 48, p. 339.
Volfkovich, Yu.M., Blinov, I.A., and Sakar, A., US Patent 7059175, 2006.
Rouquerol, J., Baron, G., Denoyel, R., Giesche, H., Groen, J., Klobes, P., Levitz, P., Neimark, A.V., Rigby, S., Skudas, R., Sing, K., Thommes, M., and Unger, K., Pure Appl. Chem., 2012, vol. 84, p. 107.
William, S., Hummers, J.R., and Offeman, R.E., J. Am. Chem. Soc., 1958, vol. 80, p. 1339.
Shulga, Yu.M., Baskakov, S.A., Smirnov, V.A., Shulga, N.Yu., Belay, K.G., and Gutsev, G.L., J. Power Sources, 2014, vol. 245, p. 33.
Baskakov, S.A., Baskakova, Y.V., Lyskov, N.V., Dremova, N.N., Irzhak, A.V., Kumar, Y., Michtchenko, A., and Shulga, Y.M., Electrochim. Acta, 2018, vol. 260, p. 557.
Wenzel, R.N., Ind. Eng. Chem., 1936, vol. 28, p. 988.
Shulga, Y.M., Melezhik, A.V., Kabachkov, E.N., Milovich, F.O., Lyskov, N.V., Irzhak, A.V., Dremova, N.N., Gutsev, G.L., Michtchenko, A., Tkachev, A.G., and Kumar, Y., Appl. Phys. A, 2019, vol. 125, p. 460.
Gupta, R.K., Malviya, M., Ansari, K.R., Lgaz, H., Chauhan, D.S., and Quraishi, M.A., Mater. Chem. Phys., 2019, vol. 236, artic. no. 121727.
Wu, C.-K., Chem. Phys. Lett., 1973, vol. 21, p. 153.
Gruger, A., Regis, A., Schmatko, T., and Colomban, P., Vib. Spectrosc., 2001, vol. 26, p. 215.
Pimenta, M.A. and Dresselhaus, G., Annu. Rep. Prog. Chem. Sect. C: Phys. Chem., 2007, vol. 9, p. 1276.
Brown, R.G., J. Chem. Phys., 1964, vol. 40, p. 2900.
Volfkovich, Yu.M., Lobach, A.S., Spitsyna, N.G., Baskakov, S.A., Sosenkin, V.E., Rychagov, A.Yu., Kabachkov, E.N., and Shulga, Yu.M., J. Porous Mater., 2019, vol. 26, p. 1111.
Gregg, S.J. and Sing, K.S.W., Adsorption, Surface Area and Porosity, New York: Academic Press, 1967.
Eikerling, M., Kharkats, Y.I., Kornyshev, A.A., and Volfkovich, Y.M., J. Electrochem. Soc., 1998, vol. 145, p. 2684.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, London: Wiley, 2001.
ACKNOWLEDGMENTS
This work was performed using the equipment of the Analytical Center for Collective Use of the Institute of Problems of Chemical Physics of the Russian Academy of Sciences, and Chernogolovka Research Center and partly implemented using the resources of the Center of Competences of National Technological Initiative of the Institute of Problems of Chemical Physics, Russian Academy of Sciences.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the State tasks АААА-А19-119041890032-6, АААА-А19-119032690060-9, and АААА-А19-119061890019-5.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Volfkovich, Y.M., Sosenkin, V.E., Maiorova, N.A. et al. Graphene-Based Aerogels Possessing Superhydrophilic and Superhydrophobic Properties and Their Application for Electroreduction of Molecular Oxygen. Colloid J 83, 284–293 (2021). https://doi.org/10.1134/S1061933X21030157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X21030157