Abstract
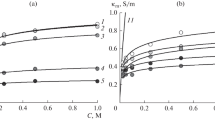
The possibility of calculating the ionic composition of a membrane and the ion-exchange equilibrium constant on the basis of the concentration dependences of the specific conductivity of the membrane in individual and mixed solutions has been studied for an ion-exchange membrane–solution system, in which the solution contains two types of counterions and a common coion. The calculation has been carried out for MF-4SK and MK-40 membranes in a mixed solution of calcium and sodium chlorides, and a satisfactory agreement of the obtained values with those reported in the literature has been shown. This approach has been used to estimate the ion-exchange equilibrium constant for polyaniline-modified MF-4SK and MK-40 membranes, and a correlation has been shown between the results of the conductivity studies and the assessments of the counterion fluxes during the electrodialysis of the mixed solutions.



Similar content being viewed by others
REFERENCES
Sata, T. and Yang, W., J. Membr. Sci., 2002, vol. 206, p. 31.
Luo, T., Roghmans, F., and Wessling, M., J. Membr. Sci., 2020, vol. 597, p. 117645.
Luo, T., Abdu, S., and Wessling, M., J. Membr. Sci., 2018, vol. 555, p. 429.
Zabolotskii, V.I., Gnusin, N.P., Reprintseva, S.L., and Nikonenko, V.V., Elektrokhimiya, 1979, vol. 15, p. 1124.
Zabolotsky, V.I., Achoh, A.R., Lebedev, K.A., and Melnikov, S.S., J. Membr. Sci., 2020, vol. 608, artic. no. 118152.
Zolotukhina, E.V. and Kravchenko, T.A., Zh. Fiz. Khim., 2001, vol. 83, p. 934.
Mahmoud, A. and Hoadley, A.F.A., Water Res., 2012, vol. 46, p. 3364.
Gnusin, N.P., Karpenko, L.V., Demina, O.A., and Berezina, N.P., Zh. Fiz. Khim., 2001, vol. 75, p. 1697.
Karpenko-Jereb, L.V. and Berezina, N.P., Desalination, 2009, vol. 245, p. 587.
Karavanova, Yu.A. and Yaroslavtsev, A.B., Inorg. Mater., 2010, vol. 46, p. 789.
Karavanova, Yu.A., Fedina, K.G., and Yaroslavtsev, A.B., Inorg. Mater., 2011, vol. 47, p. 329.
Helfferich, F.G., Ionenaustauscher, Weinheim: Verlag Chemie, 1959.
Zabolotsky, V.I. and Nikonenko, V.V., J. Membr. Sci., 1993, vol. 79, p. 181.
Berezina, N.P., Kononenko, N.A., Dyomina, O.A., and Gnusin, N.P., Adv. Colloid Interface Sci., 2008, vol. 139, p. 3.
Loza, N.V., Loza, S.A., Kononenko, N.A., and Magalyanov, A.V., Membr. Membr. Tekhnol., 2015, vol. 5, p. 202.
Demina, O.A., Falina, I.V., Kononenko, N.A., and Zabolotskiy, V.I., Colloid J., 2020, vol. 82, p. 108.
Golubenko, D. and Yaroslavtsev, A., J. Membr. Sci., 2020, vol. 612, artic. no. 118408.
Tan, S., Tieu, J.H., and Belanger, D., J. Phys. Chem. B, 2005, vol. 109, p. 14085.
Nagarale, R.K., Gohil, G.S., Shahi, V.K., Trivedi, G.S., and Rangarajan, R., J. Colloid Interface Sci., 2004, vol. 277, p. 162.
Shkirskaya, S.A., Senchikhin, I.N., Kononenko, N.A., and Roldugin, V.I., Russ. J. Electrochem., 2017, vol. 53, p. 78.
Golubenko, D.V., Karavanova, Yu.A., Melnikov, S.S., Achoh, A.R., Pourcelly, G., and Yaroslavtsev, A.B., J. Membr. Sci., 2018, vol. 563, p. 777.
Zabolotskii, V.I. and Nikonenko, V.V., Perenos ionov v membranakh (Ion Transport in Membranes), Moscow: Nauka, 1996.
Kononenko, N.A., Dolgopolov, S.V., Loza, N.V., and Shel’deshov, N.V., Russ. J. Electrochem., 2015, vol. 51, p. 19.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-38-20 069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Falina, I.V., Kononenko, N.A., Demina, O.A. et al. Estimation of Ion-Exchange Equilibrium Constant Using Membrane Conductivity Data. Colloid J 83, 379–386 (2021). https://doi.org/10.1134/S1061933X21030054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X21030054