Effect of the Forest-Mine Boundary Form on Woody Colonization and Forest Expansion in Degraded Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Sampling
2.3. Data Analysis
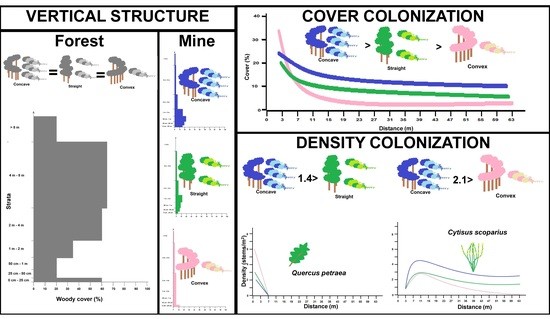
3. Results
3.1. Vertical Structure and Composition of Woody Vegetation in the Forest Edge
3.2. Vertical Structure of Woody Vegetation in the Mines
3.3. Boundary Form Effect on Total Woody Colonization (Density) in the Mines
3.4. Boundary Form Effect on Main Woody Colonizers of Mines
3.5. Influence of Browsing
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Species | ≥2 m ≥10 cm dbh | ≥2 m <10 cm dbh | ≥60 cm y < 2 m <10 cm dbh | ≥30 cm y < 60 cm <10 cm dbh | <30 cm <10 cm dbh |
|---|---|---|---|---|---|
| Quercus petraea (Matt.) Liebl. | 100 | 62.85 | 71.89 | 27.86 | 24.63 |
| Rosa canina L. | 0 | 8.97 | 5.79 | 33.22 | 15.84 |
| Ligustrum vulgare L. | 0 | 0 | 4.72 | 8.30 | 3.52 |
| Crataegus monogyna Jacq. | 0 | 12.82 | 2.87 | 6.27 | 12.32 |
| Rubus ulmifolius Schott. | 0 | 0 | 3.83 | 8.49 | 3.23 |
| Cytisus scoparius (L.) Link | 0 | 1.28 | 1.44 | 4.06 | 19.36 |
| Genista florida L. | 0 | 11.54 | 0.59 | 1.85 | 7.04 |
| Euonymus europaeus L. | 0 | 1.28 | 1.47 | 1.29 | 1.76 |
| Ilex aquifolium L. | 0 | 1.28 | 0.59 | 0.92 | 0.29 |
| Lonicera xylosteum L. | 0 | 0 | 0.15 | 0.55 | 0.29 |
| Lonicera periclymenum L. | 0 | 0 | 0 | 0 | 0.29 |
| Sorbus aria (L.) Crantz | 0 | 0 | 0.26 | 0 | 0.59 |
| Erica arborea L. | 0 | 0 | 4.39 | 4.61 | 7.04 |
| Prunus spinosa L. | 0 | 0 | 0.18 | 0.92 | 2.64 |
| Malus sylvestris Mill. | 0 | 0 | 0.37 | 1.66 | 1.17 |
| Vaccinium myrtillus L. | 0 | 0 | 1.40 | 0 | 0 |
| Viburnum lantana L. | 0 | 0 | 0.07 | 0 | 0 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Model | d.f. | AIC | ΔAIC | p | |
|---|---|---|---|---|---|
| (1) Quercus petraea | |||||
| Global | Null | 3 | 975.11 | 75.56 | |
| Lineal | 8 | 977.55 | 78.00 | <0.0001 | |
| Exp. decay | 8 | 899.55 | 0.00 | <0.0001 | |
| Boundary type | |||||
| Concave | Null | 3 | 295.97 | 39.33 | |
| Lineal | 4 | 296.71 | 40.07 | 0.2629 | |
| Exp. decay | 4 | 256.64 | 0.00 | <0.0001 | |
| Convex | Null | 3 | 317.87 | 100.67 | |
| Lineal | 4 | 309.63 | 92.43 | 0.0014 | |
| Exp. decay | 4 | 217.20 | 0.00 | <0.0001 | |
| Straight | Null | 16 | 333.11 | 16.49 | |
| Lineal | 338.49 | 21.87 | 0.0662 | ||
| Exp. decay | 316.62 | 0.00 | <0.001 | ||
| (2) Cytisus scoparius | |||||
| Global | Null | 3 | 1729,98 | 35.54 | |
| Lineal | 8 | 1736.56 | 50.91 | 0.6360 | |
| Exp. growth | 8 | 1685.65 | 0.00 | <0.0001 | |
| Boundary type | |||||
| Concave | Null | 3 | 736.64 | 13.23 | |
| Lineal | 4 | 745.23 | 21.82 | 0.0102 | |
| Exp. growth | 4 | 723.41 | 0.00 | <0.0001 | |
| Convex | Null | 3 | 429.86 | 5.19 | |
| Lineal | 4 | 429.98 | 5.31 | 0.1693 | |
| Exp. growth | 4 | 424.67 | 0.00 | 0.0073 | |
| Straight | Null | 16 | 502.35 | 5.86 | |
| Lineal | 510.26 | 13.77 | 0.015 | ||
| Exp. growth | 496.49 | 0.00 | 0.005 | ||
| (3) Genista florida | |||||
| Global | Null | 3 | 1401.70 | 13.00 | |
| Lineal | 4 | 1432.27 | 43.57 | 0.001 | |
| Exp. decay | 4 | 1388.70 | 0.00 | 0.0003 | |
| (4) Crataegus monogyna | |||||
| Global | Null | 3 | 513.05 | 5.27 | |
| Lineal | 4 | 538.34 | 30.58 | 0.0092 | |
| Exp. decay | 4 | 507.78 | 0.00 | <0.0001 | |
| Boundary type | |||||
| Concave | Null | 3 | 290.07 | 20.88 | |
| Lineal | 4 | 294.85 | 25.66 | 0.0956 | |
| Exp. decay | 4 | 269.19 | 0.00 | 0.0001 | |
| Convex | Null | 3 | 40.18 | 14.4 | |
| Lineal | 4 | 42.73 | 16.95 | 0.4584 | |
| Exp. decay | 4 | 25.78 | 0.00 | 0.0001 | |
| Straight | Null | 16 | 83.15 | −9.15 | |
| Lineal | 96.20 | −13.05 | 0.0642 | ||
| Exp. Decay | 92.30 | 0.00 | 0.0008 | ||
| (5) Rosa canina | |||||
| Global | Null | 3 | 534.30 | 10.22 | |
| Lineal | 4 | 577.09 | 53.01 | <0.0001 | |
| Exp. decay | 4 | 524.08 | 0.00 | 0.0011 | |
| (6) Rubus ulmifolius | |||||
| Global | Null | 3 | 544.33 | 17.76 | |
| Lineal | 4 | 575.18 | 48.61 | 0.0009 | |
| Exp. decay | 4 | 526.57 | 0.00 | <0.0001 |
| Model | d.f. | AIC | ΔAIC | p | |
|---|---|---|---|---|---|
| Global | |||||
| Null | 3 | 1999.93 | 57.41 | ||
| Lineal | 8 | 2002.52 | 60.00 | 0.192 | |
| Exp. decay | 8 | 1942.52 | 0.00 | <0.0001 | |
| Boundary type | |||||
| Concave | Null | 3 | 792.76 | 24.75 | |
| Lineal | 4 | 797.77 | 29.76 | 0.008 | |
| Exp. decay | 4 | 768.01 | 0.00 | <0.0001 | |
| Convex | Null | 3 | 644.06 | 16.8 | |
| Lineal | 4 | 641.37 | 14.11 | 0.0304 | |
| Exp. decay | 4 | 627.26 | 0.00 | <0.0001 | |
| Straight | Null | 16 | 545.25 | 8.05 | |
| Lineal | 551.61 | 14.41 | 0.0368 | ||
| Exp. decay | 537.20 | 0.00 | 0.0015 |
References
- Forman, R.T.T.; Moore, P.N. Theoretical foundations for understanding boundaries. In Landscape Boundaries: Consequences for Biotic Diversity and Ecological Flows; Hansen, A.J., di Castri, F., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Forman, R.T.T. Land Mosaics: The Ecology of Landscape and Regions; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Hardt, R.A.; Forman, R.T.T. Boundary form effects on woody colonization of reclaimed surface mines. Ecology 1989, 70, 1252–1260. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Godron, M. Landscape Ecology; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Forman, R.T.T. Emerging directions in landscape ecology and applications in natural resource management. In Proceedings of the Conference on Science in the National Parks; Herrmann, R., Bostedt-Craig, T., Eds.; US National Park Service and the George Wright Society: Fort Collins, CO, USA, 1986; pp. 59–88. [Google Scholar]
- López-Barrera, F.; Manson, R.H.; González-Espinosa, M.; Newton, A. Effects of varying forest edge permeability on seed dispersal in a neotropical montane forest. Landsc. Ecol. 2007, 22, 189–203. [Google Scholar] [CrossRef]
- Milder, A.I.; Fernández-Santos, B.; Martínez-Ruiz, C. Colonization patterns of woody species on lands mined for coal in Spain: Preliminary insight for forest expansion. Land Degrad. Develop. 2013, 24, 39–46. [Google Scholar] [CrossRef]
- Stamps, J.A.; Buechner, M.; Krishnan, V.V. The effects of edge permeability and habitat geometry on emigration from patches of habitat. Am. Nat. 1987, 129, 533–552. [Google Scholar] [CrossRef]
- López-Barrera, F. Estructura y función en bordes de bosques. Ecosistemas 2004, 1, 55–68. Available online: https://www.revistaecosistemas.net/index.php/ecosistemas/article/view/581 (accessed on 13 December 2020).
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef] [Green Version]
- López-Barrera, F.; Newton, A.C.; Manson, R.H. Edge effects in a tropical mountains forest mosaic: Experimental tests of post-dispersal acorn removal. Ecol. Restor. 2005, 20, 31–40. [Google Scholar] [CrossRef]
- Cadenasso, M.L.; Pickett, S.T.A. Linking forest edge structure to edge function: Mediation of herbivore damage. J. Ecol. 2000, 88, 31–44. [Google Scholar] [CrossRef]
- Cadenasso, M.L.; Pickett, S.T.A. Effect of edge structure on the flux of species into forest interiors. Conserv. Biol. 2001, 15, 91–97. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Esseen, P.A. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- McQuilkin, W.R. The natural establishment of pine in abandoned fields in the Piedmont Plateau Region. Ecology 1940, 21, 135–147. [Google Scholar] [CrossRef]
- Hatton, T.J.; Carpenter, A.T. An empirical test of the mass effect determinant of species richness. Vegetatio 1986, 68, 33–36. [Google Scholar] [CrossRef]
- González-Alday, J.; Marrs, R.; Martínez-Ruiz, C. Soil seed bank formation during early revegetation after hydroseeding in reclaimed coal wastes. Ecol. Eng. 2009, 35, 1062–1069. [Google Scholar] [CrossRef]
- Parmenter, R.R.; MacMahon, J.A.; Waaland, M.E.; Steube, M.M.; Crisafulli, C.M. Reclamation of surface coal mines in western Wyoming for wildlife habitat. Reclam. Reveg. Res. 1985, 4, 93–115. [Google Scholar]
- Gómez, J.M.; García, D.; Zamora, R. Impact of vertebrate acorn- and seedling-predators on a Mediterranean Quercus pyrenaica forest. For. Ecol. Manag. 2003, 180, 125–134. [Google Scholar] [CrossRef]
- Torroba-Balmori, P.; Zaldívar, P.; Alday, J.G.; Fernández-Santos, B.; Martínez-Ruiz, C. Recovering Quercus species on reclaimed coal wastes using native shrubs as restoration nurse plants. Ecol. Eng. 2015, 77, 146–153. [Google Scholar] [CrossRef]
- Costa, A.; Villa, S.; Alonso, P.; García-Rodríguez, J.A.; Martín, F.J.; Martínez-Ruiz, C.; Fernández-Santos, B. Can native shrubs facilitate the early establishment of contrasted co-occurring oaks in Mediterranean grazed areas? J. Veg. Sci. 2017, 28, 1047–1056. [Google Scholar] [CrossRef]
- Callaway, R.M. Effect of shrub on recruitment of Quercus douglasii and Quercus lobata in California. Ecology 1992, 73, 2118–2128. [Google Scholar] [CrossRef]
- Rousset, O.; Lepart, J. Positive and negative interactions at different life stages of a colonizing species (Quercus humilis). J. Ecol. 2000, 88, 401–412. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Prach, K.; Hobbs, R.J. Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Prach, K.; Walker, L.R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 2011, 26, 119–123. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Caracterización Agroclimática de la Provincia de Palencia; Ministerio de Agricultura Pesca y Alimentación: Madrid, Spain, 1991. [Google Scholar]
- Sigcha, F.; Pallavicini, Y.; Camino, M.J.; Martínez-Ruiz, C. Effects of short-term grazing exclusion on vegetation and soil in early succession of a Subhumid Mediterranean reclaimed coal mine. Plant Soil 2018, 426, 197–209. [Google Scholar] [CrossRef]
- Soil-Survey-Staff. Keys to Soil taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, WA, USA, 2014. [Google Scholar] [CrossRef]
- López-Marcos, D.; Turrión, M.B.; Martínez-Ruiz, C. Linking soil variability with plant community composition along a mine-slope topographic gradient: Implications for restoration. Ambio 2020, 49, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Richards, A. Testing ecological theory using the informationtheoretic approach: Examples and cautionary results. Ecology 2005, 86, 2805–2814. [Google Scholar] [CrossRef]
- Akaike, H. Information theory as an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-Plus; Springer: New York, NY, USA, 2000. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- González-Alday, J.; Marrs, R.; Martínez-Ruiz, C. The influence of aspect on the early growth dynamics of hydroseeded species in coal reclamation areas. Appl. Veg. Sci. 2008, 11, 405–412. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: http://www.r-project.org (accessed on 1 February 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R-Core-Team. Package ‘‘nlme.’’ nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-137; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 February 2020).
- Valladares, F.; Balaguer, L.; Martínez Ferri, E.; Pérez-Corona, E.; Manrique, E. Plasticity, instability and canalization: Is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems? New Phytol. 2002, 156, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Chambel, M.R.; Climent, J.; Alia, R.; Valladares, F. Phenotypic plasticity: A useful framework for understanding adaptation in forest species. Investig. Agrar. Sist. Recur. For. 2005, 14, 334–344. [Google Scholar] [CrossRef]
- Tárrega, R.; Calvo, L.; Trabaud, L. Effect of high temperatureas on seed germination of two woody Leguminosae. Vegetatio 1992, 102, 139–147. [Google Scholar] [CrossRef]
- Alday, J.G.; Zaldívar, P.; Torroba-Balmori, P.; Fernández-Santos, B.; Martínez-Ruiz, C. Natural forest expansion on reclaimed coal mines in Northern Spain: The role of native shrubs as suitable microsites. Environ. Sci. Pollut. Res. 2016, 23, 13606–13616. [Google Scholar] [CrossRef]
- Hoshovsky, M. Element stewardship abstract for Cytisus scoparius and Genista monspessulana. In Scotch Broom, French Broom; The Nature Conservancy: Arlington, VA, USA, 1986. [Google Scholar]
- Malo, J.E. Potential ballistic dispersal of Cytisus scoparius (Fabaceae) seeds. Austral. J. Bot. 2004, 52, 653–658. [Google Scholar] [CrossRef]
- Moreno-Marcos, G.; Gómez-Gutiérrez, J.M.; Fernández-Santos, B. Primary dispersal of Cytisus multiflorus seeds. Pinineos 1992, 140, 75–88. [Google Scholar] [CrossRef]
- Malo, J.E.; Suárez, F. Herbivorous mammals as seed disperser in Mediterranean dehesa. Am. Nat. 1995, 123, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Osoro, K.; Celaya, R.; Martínez, A.; Zorita, E. Pastoreo de las comunidades vegetales de montaña por rumiantes domésticos: Producción animal y dinámica vegetal. Pastos 2000, 30, 3–50. [Google Scholar]
- Milder, A.I. Study of Mechanisms that Promote Forest Restoration in Degraded Areas: Facilitation, Forest Edge Shape and Propagation Strategies. Ph.D. Thesis, University of Salamanca, Salamanca, Spain, 2016. [Google Scholar]
- Bauer, H.J. Ten years’ studies of biocenological succession in the excavated mines in the Cologne district. In Ecology and Reclamation of Devastated Land; Hutnik, R.J., Davis, G., Eds.; Gordon and Breach: New York, NY, USA, 1973; pp. 271–284. [Google Scholar]
- Peterson, D.J.; Prasard, R. The biology of Canadian weeds. 109. Cytisus scoparius (L.) Link. Can. J. Plant Sci. 1998, 78, 497–504. [Google Scholar] [CrossRef]
- Fogarty, G.; Facelli, J.M. Growth and competition of Cytisus scoparius, an invasive shrub, and Australian native shrubs. Plant Ecol. 1999, 144, 27–35. [Google Scholar] [CrossRef]
- Cruz, O.; García-Duro, J.; Riveiro, S.F.; García-García, C.; Casal, M.; Reyes, O. Fire severity drives the natural regeneration of Cytisus scoparius L.(Link) and Salix atrocinerea Brot. Communities and the germinative behaviour of these Species. Forests 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Calcerrada, J.; Pardos, J.A.; Gil, L.; Reich, P.B.; Aranda, I. Light response in seedlings of a temperate (Quercus petraea) and a sub-Mediterranean species (Quercus pyrenaica): Contrasting ecological strategies as potential keys to regeneration performance in mixed marginal populations. Plant Ecol. 2008, 195, 273–285. [Google Scholar] [CrossRef]
- Do Amaral Franco, J.; Quercus, L. Flora Iberica; Castro, S., Laínz, M., López González, G., Montserrat, P., Muñoz Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 1990; Volume 2, pp. 15–36. [Google Scholar]
- Olthoff, A.; Martínez-Ruiz, C.; Alday, J.G. Distribution patterns of forest species along an Atlantic-Mediterranean environmental gradient: An approach from forest inventory data. Forestry 2016, 86, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.M.; Puerta-Piñero, C.; Schupp, E.W. Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 2008, 155, 529–537. [Google Scholar] [CrossRef]
- Müller, S.C.; Overbeck, G.E.; Pfadenhauer, J.; Pillar, V.D. Plant functional types of woody species related to fire disturbance in forest-grassland ecotones. Plant Ecol. 2007, 189, 1–14. [Google Scholar] [CrossRef]
- Díaz-Hernández, R.; Vicente Villardón, J.L.; Martínez-Ruiz, C.; Fernández-Santos, B. The effects of native shrub, fencing, and acorn size on the emergence of contrasting co-occurring oaks in Mediterranean grazed areas. Forests 2021, 12, 307. [Google Scholar] [CrossRef]
- Alday, J.G.; Santana, V.M.; Marrs, R.H.; Martínez-Ruiz, C. Shrub-induced understory vegetation changes in reclaimed mine sites. Ecol. Eng. 2014, 73, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Burschel, P.; Huss, J. Grundriß des Waldbaus; Parey: Berlin, Germany, 2003. [Google Scholar]
- Carlo, T.A.; García, D.; Martínez, D.; Morales, J.M. Where do seeds go when they go far? Integrating distance and directionality of avian seed dispersal in heterogeneous landscapes. Ecology 2013, 94, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Padilla, M. Ecogenetic Study of Plant Populations in Altered Media: Coal Mining. Ph.D. Thesis, University of León, León, Spain, 1993. [Google Scholar]
- Williams-Linera, G. Vegetation structure and environmental conditions of forest edges in Panama. J. Ecol. 1990, 78, 356–373. [Google Scholar] [CrossRef]
- Martínez-Ruiz, C.; Marrs, R.H. Some factors affecting successional change on uranium mine wastes: Insights for ecological restoration. Appl. Veg. Sci. 2007, 10, 333–342. [Google Scholar] [CrossRef]
- Martín-Sanz, R.C.; Fernández-Santos, B.; Martínez-Ruiz, C. Early dynamics of natural revegetation on roadcuts of the Salamanca province (CW Spain). Ecol. Eng. 2015, 75, 223–231. [Google Scholar] [CrossRef]
- Schmitz, O.J. Scaling from plot experiments to landscapes: Studying grasshoppers to inform forest ecosystem management. Oecologia 2005, 145, 225–234. [Google Scholar] [CrossRef]
- Rothley, K.D. Use of multiobjective optimization models to examine behavioural trade-offs of white-tailed deer habitat use forest harvesting experiments. Can. J. For. Res. 2002, 32, 1275–1284. [Google Scholar] [CrossRef]
- Ammar, H.; López, S.; González, J.S.; Ranilla, M.J. Seasonal variations in the chemical composition and in vitro digestibility of some Spanish leguminous shrub species. Anim. Feed Sci. Technol. 2004, 115, 327–340. [Google Scholar] [CrossRef]
- Kramer, K.; Groot Bruinderink, G.W.T.A.; Prins, H.H.T. Spatial interactions between ungulate herbivory and forest management. For. Ecol. Manag. 2006, 226, 238–247. [Google Scholar] [CrossRef]
- Bobiec, A.; Kuijper, D.P.J.; Niklasson, M.; Romankiewicz, A.; Solecka, K. Oak (Quercus robur L.) regeneration in early successional woodlands grazed by wild ungulates in the absence of livestock. For. Ecol. Manag. 2011, 262, 780–790. [Google Scholar] [CrossRef]





| df | F | p | ||
|---|---|---|---|---|
| Intercept | 1 | 2220 | 284.03 | <0.0001 |
| Boundary form | 2 | 20 | 21.51 | <0.0001 |
| Stratum | 6 | 2220 | 61.56 | <0.0001 |
| Distance | 15 | 2220 | 30.47 | <0.0001 |
| Boundary form × stratum | 12 | 2220 | 11.90 | <0.0001 |
| Boundary form × distance | 30 | 2220 | 5.00 | <0.0001 |
| Stratum × distance | 90 | 2220 | 3.14 | <0.0001 |
| Boundary form × stratum × distance | 180 | 2220 | 0.87 | 0.8833 |
| Model | d.f. | AIC | ΔAIC | p | |
|---|---|---|---|---|---|
| Global | |||||
| Null | 3 | 2030.24 | 35.54 | ||
| Lineal | 8 | 2016.43 | 21.73 | <0.0001 | |
| Exp. decay | 8 | 1994.70 | 0.00 | <0.0001 | |
| Boundary type | |||||
| Concave | Null | 3 | 802.36 | 8.49 | |
| Lineal | 4 | 807.52 | 13.65 | 0.079 | |
| Exp. decay | 4 | 793.87 | 0.00 | 0.049 | |
| Convex | Null | 3 | 608.36 | 46.85 | |
| Lineal | 4 | 598.55 | 37.04 | <0.001 | |
| Exp. decay | 4 | 561.51 | 0.00 | <0.001 | |
| Straight | Null | 3 | 612.17 | 13.10 | |
| Lineal | 4 | 612.97 | 13.90 | 0.007 | |
| Exp. decay | 4 | 599.07 | 0.00 | 0.010 |
| Best Model | Boundary Form | Distance | Boundary Form × Distance | |
|---|---|---|---|---|
| Quercus petraea | Exp. decay | 1.17 | 119.19 *** | 13.36 *** |
| Cytisus scoparius | Exp. growth | 13.47 *** | 7.23 ** | 2.57 * |
| Genista florida | Exp. decay | 0.68 | 1.58 | 0.52 |
| Crataegus monogyna | Exp. decay | 0.63 | 29.94 *** | 8.70 *** |
| Rosa canina | Exp. decay | 0.88 | 7.31 ** | 2.84 |
| Rubus ulmifolius | Exp. decay | 1.54 | 9.67 ** | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ruiz, C.; Milder, A.I.; López-Marcos, D.; Zaldívar, P.; Fernández-Santos, B. Effect of the Forest-Mine Boundary Form on Woody Colonization and Forest Expansion in Degraded Ecosystems. Forests 2021, 12, 773. https://doi.org/10.3390/f12060773
Martínez-Ruiz C, Milder AI, López-Marcos D, Zaldívar P, Fernández-Santos B. Effect of the Forest-Mine Boundary Form on Woody Colonization and Forest Expansion in Degraded Ecosystems. Forests. 2021; 12(6):773. https://doi.org/10.3390/f12060773
Chicago/Turabian StyleMartínez-Ruiz, Carolina, Ana I. Milder, Daphne López-Marcos, Pilar Zaldívar, and Belén Fernández-Santos. 2021. "Effect of the Forest-Mine Boundary Form on Woody Colonization and Forest Expansion in Degraded Ecosystems" Forests 12, no. 6: 773. https://doi.org/10.3390/f12060773