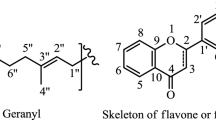
Abstract—
The study provides modern data from the literature on the results of research in the field of flavonoids of medicinal plants. Systematization was carried out according to the most common structures of the isolated 2'-hydroxychalcones and their derivatives from 59 sources of natural raw materials. The data on the features of biogenesis, general characteristics, possible functions, biological properties and aspects of practical application are presented.
Similar content being viewed by others
REFERENCES
Michael, S. and Marc, D., Epigenetic Cancer Ther., 2015, pp. 393–425. https://doi.org/10.1016/B978-0-12-800206-3.00018-5
Ismailova, G.O., Karimova, Sh.F., Ziyamutdinova, Z.K., and Bakhodirova, M. A., Al’m. Sovrem. Nauki Obraz., Tambov: Gramota, 2016, no. 10 (112), pp. 36–45.
Popova, A.V., Bondarenko, S.P., and Frasinyuk, M.S., Khim. Geterotsikl. Soedin., 2019, vol. 55, nos. 4/5, pp. 285–299.
Panasenko, A.I., Vestn. TGU, 2010, vol. 15, no. 1, pp. 62–64.
Bondakova, M.V., Formulation development and production technology of cosmetic products using grape extract, Cand. Sci. (Tech.) Dissertation, Moscow, 2014.
Gouveia, M.G., Xavier, M.A., Barreto, A.S., Gelain, D.P., Santos, J.P., Araújo, A.A., Silva, F.A., Quintans, J.S., Agra, M.F., Cabral, A.G., Tavares, J.F., Silva, M.S., and Quintans-Júnior, L.J., J. Med. Food, 2011, vol. 14, no. 11, pp. 1389–1396. https://doi.org/10.1089/jmf.2010.0212
Anderson, A., J. Am. Coll. Nutr., 2001, vol. 20, no. 4, pp. 327–336.
Stepkina, N.N. and Velikorodov, A.V., Fundam. Issled., 2015, no. 11, part 3, pp. 505–510. https://doi.org/10.17513/fr.39449
Satyanarayama, M., Tiwari, P., Tripathi, K., Srivastava, A.K., and Pratap, R., Bioorg. Med. Chem., 2004, vol. 12, pp. 883–889. https://doi.org/10.1016/j.bmc.2003.12.026
Ramirez, I., Carabot, A., Melendez, P., Carmona, J., Jimenez, M., Patel, A.V., Crabb, T.A., Blunden, G., Cary, P.D., Croft, S.L., and Costa, M., Phytochemistry, 2003, vol. 64, pp. 645–647. https://doi.org/10.1016/s0031-9422(03)00241-3
Lunardi, F., Guzela, M., Rodrigues, A.T., Corre, R., Eger-Mangrich, I., Steindel, M., Grisard, E.C., Assreuy, J., Calixto, J.B., and Santos, A.R., Antimicrob. Agents Chemother., 2003, vol. 47, pp. 1449–1451.
Fabr, P., Puani, S., Belobr, F., and Mando, A., Monoterpene derivatives of chalcone or dihydrochalcone and their use as depigmenting agents, Inventor’s Certificate, no. 0002595860, no. 216.015.509E, Otkr., Izobret., 2020.
Yinning, C., Tao, Y., Chenghai, G., Wenhao, C., and Riming, H., Molecules J., 2014, vol. 19, pp. 1432–1458. https://doi.org/10.3390/molecules19021432
Khan, S.A., Ahmed, B., and Alam, T., Pak. J. Pharm. Sci., 2006, vol. 19, pp. 290–294.
Jun, N., Hong, G., and Jun, K., Bioorg. Med. Chem., 2007, vol. 15, pp. 2396–2402. https://doi.org/10.1016/j.bmc.2007.01.017
Liu, M., Wiliarat, P., and Croft, S.L., Bioorg. Med. Chem., 2003, vol. 11, pp. 2729–2738. https://doi.org/10.1016/S0968-0896(03)00233-5
Achanta, G., Modzelewska, A., Feng, L., Khan, S.R., and Huang, P.A., Mol. Pharmacol., 2006, vol. 70, pp. 426–433. https://doi.org/10.1124/mol.105.021311
Echeverria, C., Santibanez, J.F., Donoso-Tauda, O., Escobar, C.A., and Tagle, R.R., Int. J. Mol. Sci., 2009, vol. 10, pp. 221–231. https://doi.org/10.3390/ijms10010221
Romagnoli, R., Baraldi, P.G., Carrion, M.D., Cara, C.L., Cruz-Lopez, O., and Preti, D., Bioorg. Med. Chem., 2008, vol. 16, pp. 5367–5376. https://doi.org/10.1016/j.bmc.2008.04.026
Meng, C.Q., Zheng, X.S., Ni, L., Ye, Z., Simpson, J.E., Worsencroft, K.J., Hotema, M.R., Weingarten, M.D., Skudlarek, J.W., Gilmore, J.M., Hoong, L.K., Hill, R.R., Marino, E.M., Suen, K.L., Kunsch, C., Wasserman, M.A., and Sikorski, J.A., Bioorg. Med. Chem. Lett., 2004, vol. 14, pp. 1513–1517. https://doi.org/10.1016/j.bmcl.2004.01.021
Nam, N.H., Kim, Y., You, Y.J., Hong, D.H., Kim, H.M., and Ahn, B.Z., Eur. J. Med. Chem., 2003, vol. 38, pp. 179–187. https://doi.org/10.1016/S0223-5234(02)01443-5
Sabzevarib, O., Galati, G., Moridani, M.Y., Siraki, A., and O’Brien, P.J., Chem.–Biol. Interact., 2004, vol. 148, pp. 57–67.
Begum, N.A., Roy, N., Laskar, R.A., and Roy, K., Med. Chem. Res., 2011, vol. 20, pp. 184–191. https://doi.org/10.1007/s00044-010-9305-6
Barford, L., Kemp, K., Hansen, M., and Kharazmi, A., Int. Immunopharmacol., 2002, vol. 2, pp. 545–550.
Najafian, M., Ebrahim-Habibi, A., Hezareh, N., Yaghmaei, P., Parivar, K., and Larijani, B., Mol. Biol. Rep., 2010, vol. 10, pp. 271–274.
Zarghi, A., Zebardast, T., Hakimion, F., Shirazi, F.H., Rao, P.N.P., and Knaus, E.E., Bioorg. Med. Chem., 2006, vol. 14, pp. 7044–7050. https://doi.org/10.1016/j.bmc.2006.06.022
Chimenti, F., Fioravanti, R., Bolasco, A., Chimenti, P., Secci, D., Rossi, F., Yanez, M., Francisco, O.F., Ortuso, F., and Alcaro, S., J. Med. Chem., 2009, vol. 49, pp. 4912–4925.
Khatib, S., Nerua, O., Musa, R., Shmnell, M., Tamir, S., and Vaya, J., Bioorg. Med. Chem., 2005, vol. 13, pp. 433–441.
Bruno, B., Alberto, V., Pilar, M., Domenico, M., and Giuliano, D.M., Curr. Med. Chem., 2005, no. 6, pp. 713–739. https://doi.org/10.2174/0929867053202241
Hatano, T., Kusudo, M., Inada, K., Ogawa, T., Shiota, S., Tsuchiya, T., and Yoshida, T., Phytochemistry, 2005, vol. 66, pp. 2047–2055.
Shmuilovich, K., Interaction of polyfluorinated chalcones with binucleophilic reagents, Cand. Sci. (Chem.) Dissertation, Novosibirsk, 2014.
Tiwari, K.N., Monserrat, J.-P., Arnaud, HequetA., Ganem-Elbaz, C., Cresteil, T., Jaouen, G., Vessieres, A., Hillard, E.A., and Jolivalt, C., Dalton Trans., 2012, vol. 41, pp. 6451–6457.
Bhatia, N.M., Mahadik, K.R., and Bhatia, M.S., Chem. Pap., 2009, vol. 63, pp. 456–463. https://doi.org/10.2478/s11696-009-0026-6
Hamdi, N., Fischmeister, C., Puerta, M.C., and Valerga, P., Med. Chem. Res., 2011, vol. 20, pp. 522–530. https://doi.org/10.1007/s00044-010-9326-1
Bonakdar, A.P.S., Sadeghi, A., Aghaei, H.R., Beheshtimaal, K., Nazifi, S.M.R., and Massah, A.R., Russ. J. Bioorg. Chem., 2020, vol. 46, pp. 371–381. https://link.springer.com/journal/11171/46/3.
Dao, T.T., Nguyen, P.H., Lee, H.S., Kim, E., Park, J., Lim, S., and Oh, W.K., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 294–298. https://doi.org/10.1016/j.bmcl.2010.11.016
Yenesew, A., Induli, M., Derese, S., Midiwo, J.O., Heydenreich, M., Peter, M.G., Akala, H., Wangui, J., Liyala, P., and Waters, N.C., Phytochemistry, 2004, vol. 65, pp. 3029–3032. https://doi.org/10.1016/j.phytochem.2004.08.050
Portet, B., Fabre, N., Roumy, V., Gornitzka, H., Bourdy, G., Chevalley, S., Sauvain, M., Valentin, A., and Moulis, C., Phytochemistry, 2007, vol. 68, pp. 1312–1320. https://doi.org/10.1016/j.phytochem.2007.02.006
Yenesew, A., Derese, S., Midiwo, J.O., Oketch-Rabah, H.A., Lisgarten, J., Palmer, R., Heydenreich, M., Peter, M.G., Akala, H., Wangui, J., Liyala, P., and Waters, N.C., Phytochemistry, 2003, vol. 64, pp. 773–779. https://doi.org/10.1016/s0031-9422(03)00373-x
Jayasinghe, L., Balasooriya, B.A.I.S., Padmini, W.C., Hara, N., and Fujimoto, Y., Phytochemistry, 2004, vol. 65, pp. 1287–1290.
Epifano, F., Genovese, S., Menghini, L., and Curini, M., Phytochemistry, 2007, vol. 68, pp. 939–953. https://doi.org/10.1016/j.phytochem.2007.01.019
Jayasinghe, L., Rupasinghe, G., Hara, N., and Fujimoto, Y., Phytochemistry, 2006, vol. 67, pp. 1353–1358.
Apak, R., Guclu, K., Demirata, B., Ozyurek, M., Celik, S.E., Bektasoglu, B., Berker, K.I., and Ozyurt, D., Molecules J., 2007, vol. 12, pp. 1496–1547. https://doi.org/10.3390/12071496
Bajgai, E.S., Prachyawarakarn, V., Mahidol, C., Ruchirawat, S., and Kittakoop, P., Phytochemistry, 2011, vol. 72, pp. 2062–2067.
Stevens, J.F. and Page, J.E., Phytochemistry, 2004, vol. 65, pp. 1317–1330. https://doi.org/10.1016/j.phytochem.2004.04.025
Prachayasittikul, S., Buraparuangsang, P., Worachartcheewan, A., Isarankura-Na-Ayudhya, C., Ruchirawat, S., and Prachayasittikul, V., Molecules J., 2008, vol. 13, pp. 904–921. https://doi.org/10.3390/molecules13040904
Kurkin V.A., Kurkina A.V., Avdeeva E.V., Ratsion. Pitan., Pishch. Dobavki Biostimulyatory, 2014, no. 4, p. 26.
Nerya, O., Musa, R., Khatib, S., Tamir, S., and Vaya, J., Phytochemistry, 2004, vol. 65, pp. 1389–1395. https://doi.org/10.1016/j.phytochem.2004.04.016
Calliste, C.A., Le Bail, J.C., Trouilas, P., Pouget, C., Habrioux, G., and Chulia, A.J., Anticancer Res., 2001, vol. 21, pp. 3949–3956.
Bo, Han., Zheng, Q., Wang, J., Chen, W., Tang, H., Qi, Wang., Wang, X., and Ji, Li., Khim. Prir. Soedin., 2010, no. 4, pp. 443–446.
Martinez-Luis, S., Perez-Vasquez, A., and Mata, R., Phytochemistry, 2007, vol. 68, pp. 1882–1903. https://doi.org/10.1016/j.phytochem.2007.02.025
Meazza, G., Scheffler, B.E., Tellez, M.R., Rimando, A.M., Romagni, J.G., Duke, S.O., Nanayakkara, D., Khan, I.A., Abourashed, E.A., and Dayan, F.E., Phytochemistry, 2002, vol. 59, pp. 281–288.
Haraguchi, H., Tanaka, Y., Kabbash, A., Fujioka, T., Ishizu, T., and Yagi, A., Phytochemistry, 2004, vol. 65, pp. 2255–2260. https://doi.org/10.1016/s0031-9422(03)00516-8
Eckermann, C., Matthes, B., Nimtz, M., Reiser, V., Lederer, B., Boger, P., and Schroder, J., Phytochemistry, 2003, vol. 64, pp. 1045–1054. https://doi.org/10.1016/s0031-9422(03)00516-8
Bastos dos Santos, M., Bertholin Anselmo, D., Gisleine de Oliveira, J., Jardim-Perassi, B.V., et al., Antiproliferative activity and p53 upregulation effects of chalcones on human breast cancer cells, J. Enzyme Inhib. Med. Chem., 2019, pp. 1093–1099. https://doi.org/10.1080/14756366.2019.1615485
Hsieh, H.K., Tsao, L.T., Wang, J.P., and Lin, C.N., J. Pharm. Pharmacol., 2000, vol. 52, pp. 163–171. https://doi.org/10.1211/0022357001773814
Won, S.J., Liu, C.T., Tsao, L.T., Weng, J.R., Ko, H.H., Wang, J.P., and Lin, C.N., Eur. J. Med. Chem., 2005, vol. 40, pp. 103–112. https://doi.org/10.1016/j.ejmech.2004.09.006
Okunade, A.L., Elvin-Lewis, M.P.F., and Lewis, W.H., Phytochemistry, 2004, vol. 65, pp. 1017–1032. https://doi.org/10.1016/j.phytochem.2004.02.013
Aitmambetov, A., Ibragimova, Z.Yu., Khozhambergenov, K., and Ibragimov, M.Yu., Teoret. Klin. Med. (Tashkent), 2006, no. 5, p. 184.
Hyun, AhJ., Takako, Y., Byung-Woo, K., Jee, H.J., and Jae, S.Ch., Am. J. Chin. Med., 2010, no. 2, pp. 415–429. https://doi.org/10.1142/S0192415X10007944
Hongyu, W., Tingting, L., and Dejian, H., Adv. Food Nutr. Res., 2013, vol. 70, pp. 103–136. https://doi.org/10.1016/B978-0-12-416555-7.00003-5
Yue, Y., Xuchao, J., Haihui, X., and Xiaoyi, W., Phytochemistry, 2020, p. 174. https://doi.org/10.1016/j.phytochem.2020.112364
Toshihiro, A., Harukuni, T., Motohiko, U., Masao, I., Stefan, S., Kazuya, O., Teruo, M., Kenji, I., Takashi, S., and Hoyoku, N., Cancer Lett., 2003, vol. 201, no. 2, pp. 133–137. https://doi.org/10.1016/S0304-3835(03)00466-X
Rosa, M.P.G., Alethia, M-R., and Jahel, V.S., Afr. J. Pharm. Pharmacol., 2015, vol. 9, no. 8, pp. 237–257. https://doi.org/10.5897/AJPP2015.4267
Hyung, W.R., Mi, H.P., Ok-Kyoung, K., Doo-Young, K., Jung-Yeon, H., Yang, H.Jo., Kyung-Seop, A., and Bang, Y.H., Bioorg. Chem., 2019, vol. 92, p. 103233. https://doi.org/10.1016/j.bioorg.2019.103233
Tamires, C.L., Rafaela, J.S., Alan, D.C.S., Milene, H.M., Nicole, E.B., Andersson, B., Mário, S., and Maique, W.B., Nat. Prod. Res., vol. 30, no. 5, pp. 1–7. https://doi.org/10.1080/14786419.2015.1030740
Øyvind, M.A. and Monica, J., Compr. Nat. Prod. II, 2010, vol. 3, pp. 547–614. https://doi.org/10.1016/B978-008045382-8.00086-1
Dykens, J.A., Compr. Med. Chem. II, 2007, vol. 2, pp. 1053–1087. https://doi.org/10.1016/B0-08-045044-X/00071-7
Fera, K., Lia, D.J., Yana, M.S., Sjamsul, A.A., Euis, H.H., Kiyotaka, K., Kaoru, K., and Kunio, T., J. Nat. Med., 2010, vol. 64, pp. 121–125. https://doi.org/10.1007/s11418-009-0368-y
Anam Edet, M.U., Russ. Zh. Khim., 2000, vol. 2, p. 206. 19E.
Krasnov, E.A., Ermilova, E.V., Kadyrova, T.V., Raldugin, V.A., Bagryanskaya, I.Yu., Gatilov, Yu.V., Druganov, A.G., Semenov, A.A., and Tolstikov, G.A., Khim. Prir. Soed., 2000, no. 5, pp. 389–391.
Fuendjiep, V., Wandji, J., Tillequin, F., Mulholland, D.A., Budzikiewicz, H., Fomum, Z.T., Nyemba, A.M., and Koch, M., Phytochemistry, 2002, no. 8, pp. 803–806. https://doi.org/10.1016/s0031-9422(02)00108-5
Boonchoo, S., Kittisak, L., Jurgen, C., and Wolfgang, K., Phytochemistry, 2002, no. 8, pp. 943–947. https://doi.org/10.1016/s0031-9422(02)00337-0
Lim, S.H., Ha, T.Y., Ahn, J., and Kim, S., Phytomedicine, 2011, vol. 18, pp. 425–430. https://doi.org/10.1016/j.phymed.2011.02.002
oseph, A., Sandra, D., Matthias, H., Lois, M-M., Vicky, M.A., Mate, E., and Abiy, Y., Molecules, 2017, no. 2, p. 318. 10.3390/molecules2202031
Panthati, M.K., Rao, K.N.V., Sandhya, S., and David, B., Braz. J. Pharmacogn., 2012, vol. 22, pp. 1145–1154.
Motahare, B., Saba, S., and Mehrdad, I., Phytother. Res., 2019, no. 3, pp. 546–560. https://doi.org/10.1002/ptr.6265
Handbook of Dietary Phytochemicals, Xiao, J., , Eds., Springer Nature Singapore Pte Ltd., 2020. https://doi.org/10.1007/978-981-13-1745-3_10-1
Federico, G-G., Oswaldo, T-V., Gregorio, M-T., and Jose, S.C., Zeitschrift Naturforschung, 2014, nos. 7–8, pp. 579–583. https://doi.org/10.1515/znc-2002-7-805
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Translated by V. Mittova
Corresponding author: phone: +9 (9890) 356-11-47.
Rights and permissions
About this article
Cite this article
Ismailova, G.O., Yuldashev, N.M., Akbarhodjaeva, K.N. et al. Biologically Active Natural 2'-Hydroxychalcones. Russ J Bioorg Chem 47, 660–669 (2021). https://doi.org/10.1134/S1068162021030080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021030080