Abstract—
Phospholipases are enzymes of the class of hydrolases that catalyze the cleavage of bonds in phospholipids; they are found in almost all organisms. Enzymes of microbial origin are of the greatest interest. The popularity of bacterial enzymes is due to their huge variety and technological properties: high specific activity, thermal stability, and wide substrate specificity. The production of recombinant bacterial phospholipases and their improvement remain an urgent task, for which it is necessary to deepen and systematize knowledge about the enzymes of this group. This review describes the properties, structure, and mode of action of bacterial phospholipases C, which are widely used in various areas of human practice: scientific research, medicine, food, chemical industry, etc.





Similar content being viewed by others
REFERENCES
Aloulou, A., Ali, Y.B., Bezzine, S., Gargouri, Y., and Gelb, M.H., Methods Mol. Biol., 2012, vol. 861, pp. 63–85. https://doi.org/10.1007/978-1-61779-600-5_4
Litvinko, N.M., Izv. Nats. Akad. Nauk Belarusi, Ser. Khim. Nauk, 2015, no. 4, pp. 109–121.
Ulbrich-Hofmann, R., ChemBioChem, 2012, vol. 13, pp. 2148–2149. https://doi.org/10.1002/cbic.201200542
Fil'kin, C.Yu., Lipkin, A.V., and Fedorov, A.N., Usp. Biol. Khim., 2020, no. 60, pp. 369–410.
Borrelli, G.M. and Trono, D., Int. J. Mol. Sci., 2015, vol. 16, pp. 20774–20840. https://doi.org/10.3390/ijms160920774
Titball, R.W., Symp. Ser. Soc. Appl. Microbiol., 1998, vol. 27, p. 127.
Ramenskaia, G.V., Melnik, E.V., and Petukhov, A.E., Biomed. Khim., 2018, vol. 64, pp. 84–93. https://doi.org/10.18097/PBMC20186401084
Titball, R.W., Microbiol. Rev., 1993, vol. 57, pp. 347–366.
Songer, J.G., Trends Microbiol., 1997, vol. 5, pp. 156–161.
Scandella, C.J. and Kornberg, A., Biochemistry, 1971, vol. 10, pp. 4447–4456.
Richmond, G.S. and Smith, T.K., Int. J. Mol. Sci., 2011, vol. 12, pp. 588–612. https://doi.org/10.3390/ijms12010588
Murayama, K., Kano, K., Matsumoto, Y., and Sugimori, D., J. Struct. Biol., 2013, vol. 182, pp. 192–196. https://doi.org/10.1016/j.jsb.2013.02.003
Bakholdina, S.I., Tischenko, N.M., Sidorin, E.V., Isaeva, M.P., Likhatskaya, G.N., Dmitrenok, P.S., Kim, N.Yu., Chernikov, O.V., and Solov’eva, T.F., Biochemistry, 2016, vol. 81, pp. 47–57. https://doi.org/10.1134/s0006297916010053
Hanahan, D.J., Brockerhoff, H., and Barron, E.J., J. Biol. Chem., 1960, vol. 235, pp. 1917–1923.
Ishiwata, S., Dainihon Sanshi Kaiho, 1901, vol. 114, pp. 1–5.
Kohler, G.A., Brenot, A., Haas-Stapleton, E., Agabian, N., Deva, R., and Nigam, S., Biochim. Biophys. Acta, 2006, vol. 1761, pp. 1391–1399. https://doi.org/10.1016/j.bbalip.2006.09.011
Saito, K., Sugatani, J., and Okumura, T., Methods Enzymol., 1991, vol. 197, pp. 446–456. https://doi.org/10.1016/0076-6879(91)97170-4
Jiang, F., Huang, S., Imadad, K., and Li, C., Bioresour. Technol., 2012, vol. 104, pp. 518–522. https://doi.org/10.1016/j.biortech.2011.09.112
Matsumoto, Y., Mineta, S., Murayama, K., and Sugimori, D., FEBS J., 2013, vol. 280, pp. 3780–3796. https://doi.org/10.1111/febs.12366
Masayama, A., Kato, S., Terashima, T., Molgaard, A., Hhemmi, H., Yoshimura, T., and Moriyama, R., Biosci. Biotechnol. Biochem., 2010, vol. 74, pp. 24–30. https://doi.org/10.1271/bbb.90391
Taguchi, R. and Ikezawa, H., Arch. Biochem. Biophys., 1978, vol. 186, pp. 196–201.
Djordjevic, J.T., Front. Microbiol., 2010, vol. 1, pp. 1–13. https://doi.org/10.3389/fmicb.2010.00125
Pokotylo, I., Pejchar, P., Potocký, M., Kocourková, D., Krčková, Z., Ruelland, E., Kravets, V., and Martinec, J., Prog. Lipid Res., 2013, vol. 52, pp. 62–79. https://doi.org/10.1016/j.plipres.2012.09.001
Ivinskene, V.L., Entomopatogennye bakterii i ikh rol' v zashchite rastenii: sbornik nauchnykh trudov (Entomopathogenic Bacteria and Their Role in Plant Protection: Collection of Scientific Articles), Novosibirsk: VASKhNIL, Sib. Otd., 1987, pp. 57–75.
Ikezawa, H., Nakabayashi, T., Suzuki, K., Nakajima, M., Taguchi, T., and Taguchi, R., J. Biochem., 1983, vol. 93, pp. 1717–1719. https://doi.org/10.1093/oxfordjournals.jbchem.a134315
Volwerk, J.J., Koke, J.A., Wetherwax, P.B., and Griffith, O.H., FEMS Microbiol. Lett., 1989, vol. 61, pp. 237–241. https://doi.org/10.1111/j.1574-6968.1989.tb03629.x
Jenkins, M.G. and Frohman, M.A., Cell. Mol. Life Sci., 2005, vol. 62, pp. 2305–2316. https://doi.org/10.1007/s00018-005-5195-z
Selvy, P.E., Lavieri, R.R., Lindsley, C.W., and Brown, H.A., Chem. Rev., 2011, vol. 111, pp. 6064–6119. https://doi.org/10.1021/cr200296t
Sakurai, J., Nagahama, M., and Oda, M., J. Biochem., 2004, vol. 136, pp. 569–574. https://doi.org/10.1093/jb/mvh161
González-Bulnes, P., González-Roura, A., Canals, D., Delgado, A., Casas, J., and Llebaria, A., Bioorg. Med. Chem., 2010, vol. 18, pp. 8549–8555. https://doi.org/10.1016/j.bmc.2010.10.031
Otnaess, A.-B., Little, C., Sletten, K., Wallin, R., Johnsen, S., Flengsrud, R., and Prydz, H., Eur. J. Biochem., 1977, vol. 79, pp. 459–468. https://doi.org/10.1111/j.1432-1033.1977.tb11828.x
Elleboudy, N.S., Aboulwafa, M.M., and Hassouna, N.A., Asian Pacific J. Trop. Med., 2014, vol. 7, pp. 860–866. https://doi.org/10.1016/s1995-7645(14)60150-4
Hough, E., Hansen, L.K., Birknes, B., Jynge, K., Hansen, S., Hordvik, A., Little, C., Dodson, E., and Derewenda, Z., Nature, 1989, vol. 338, pp. 357–360. https://doi.org/10.1038/338357a0
Rose, A.S., Bradley, A.R., Valasatava, Y., Duarte, J.M., Prlić, A., and Rose, P.W., in Proceedings of the 21st International Conference on Web3D Technology— Web3D’16, 2016, pp. 185–186. https://doi.org/10.1145/2945292.2945324
Rose, A.S. and Hildebrand, P.W., Nucleic Acids Res., 2015, vol. 43, pp. W576–W579. https://doi.org/10.1093/nar/gkv402
Beecher, D.J. and Wong, A.C.L., Microbiology, 2000, vol. 146, pp. 3033–3039. https://doi.org/10.1099/00221287-146-12-3033
Lyu, Y., Ye, L., Xu, J., Yang, X., Chen, W., and Yu, H., Biotechnol. Lett., 2016, vol. 38, pp. 23–31. https://doi.org/10.1007/s10529-015-1962-6
Antikainen, N.M., Hergenrother, P.J., Harris, M.M., Corbett, W., and Martin, S.F., Biochemistry, 2003, vol. 42, pp. 1603–1610. https://doi.org/10.1021/bi0267285
Shinitzky, M., Friedman, P., and Haimovitz, R., J. Biol. Chem., 1993, vol. 268, pp. 14109–14115.
El-Sayed, M.Y., DeBose, C.D., Coury, L.A., and Roberts, M.F., Biochim. Biophys. Acta, 1985, vol. 837, pp. 325–335. https://doi.org/10.1016/0005-2760(85)90056-6
Martin, S.F., Follows, B.C., Hergenrother, P.J., and Trotter, B.K., Biochemistry, 2000, vol. 39, pp. 3410–3415. https://doi.org/10.1021/bi9919798
Snyder, W.R., Biochim. Biophys. Acta, 1987, vol. 920, pp. 155–160.
Benfield, A.P., Goodey, N.M., Phillips, L.T., and Martin, S.F., Arch. Biochem. Biophys., 2007, vol. 460, pp. 41–47.
Burley, S.K. and Petsko, G.A., Adv. Protein Chem., 1988, vol. 39, pp. 125–189. https://doi.org/10.1016/s0065-3233(08)60376-9
Dougherty, D.A., Science, 1996, vol. 271, pp. 163–168. https://doi.org/10.1126/science.271.5246.163
Celandroni, F., Salvetti, S., Senesi, S., and Ghelardi, E., FEMS Microbiol. Lett., 2014, vol. 361, pp. 95–103. https://doi.org/10.1111/1574-6968.12615
Sundell, S., Hansen, S., and Hough, E., Protein Eng., 1994, vol. 7, pp. 571–577. https://doi.org/10.1093/protein/7.4.571
Liao, R.Z., Yu, J.G., and Himo, F., Phys. Chem., vol. 114, pp. 2533–2540. https://doi.org/10.1021/jp910992f
Martin, S.F., Spaller, M.R., and Hergenrother, P.J., Biochemistry, 1996, vol. 35, pp. 12970–12977. https://doi.org/10.1021/bi961316
Seo, K.H. and Rhee, J.I., Biotechnol. Lett., 2004, vol. 26, pp. 1475–1479. https://doi.org/10.1023/b:bile.0000044447.15205.90
Durban, M.A., Silbersack, J., Schweder, T., Schauer, F., and Bornscheuer, U.T., Appl. Microbiol. Biotechnol., 2007, vol. 74, pp. 634–639. https://doi.org/10.1007/s00253-006-0712-z
Kent, C., Evers, A., and Haun, S.S.L., Arch. Biochem. Biophys., 1986, vol. 250, pp. 519–525.
Parkinson, E.K., Carcinogenes, 1987, vol. 8, pp. 857–860. https://doi.org/10.1093/carcin/8.6.857
Shimanouchi, T., Kawasaki, H., Fuse, M., Umakoshi, H., and Kuboi, R., Colloids Surf. B: Biointerfaces, 2013, vol. 103, pp. 75–83.
Mounts, T.L. and Nash, A.M., J. Am. Oil Chem. Soc., 1990, vol. 67, pp. 757–760. https://doi.org/10.1007/bf02540486
De Maria, L., Vind, J., Oxenboll, K.M., Svendsen, A., and Patkar, S., Appl. Microbiol. Biotechnol., 2007, vol. 74, pp. 290–300. https://doi.org/10.1007/s00253-006-0775-x
Cesarini, S., Haller, R.F., Diaz, P., and Nielsen, P.M., Biotechnol. Biofuels, 2014, vol. 7, pp. 1–12. https://doi.org/10.1186/1754-6834-7-29
Casado, V., Martin, D., Torres, C., and Reglero, G., Lipases and Phospholipases: Methods and Protocols, New York: Springer, 2012, vol. 861, pp. 495–523.
Arrigo, P.D. and Servi, S., Trends Biotechnol., 1997, vol. 15, pp. 90–96. https://doi.org/10.1016/s0167-7799(97)01012-3
Schümperli, M., Pellaux, R., and Panke, S., Appl. Microbiol. Biotechnol., 2007, vol. 75, pp. 33–45. https://doi.org/10.1007/s00253-007-0882-3
Morigaki, E., Miura, Y., Takahata, K., Tada, M., Nakajima, S., and Baba, N., J. Chem. Res., 1998, vol. 12, pp. 774–775. https://doi.org/10.1039/a804770g
Anthonsen, T., D’Arrigo, P., Pedrocchi-Fantoni, G., Secundo, F., Servi, S., and Sundby, E., J. Mol. Catal., 1999, vol. 6, pp. 125–132. https://doi.org/10.1016/s1381-1177(98)00141-6
Funding
This work was performed within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation (topic number FZMW-2020-0002, “Development of producers of recombinant enzymes for cheese making”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain a description of any research carried out by the authors of this work, with the participation of humans and animals as objects.
Conflict of Interests
The authors declare they have no conflict of interest.
Additional information
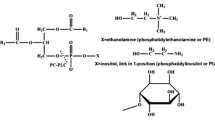
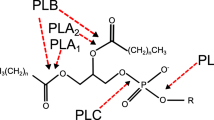
Abbreviations: PL, phospholipase; PLA1, phospholipase A1; PLA2, phospholipase A2; PLB, phospholipase B; PLC, phospholipase C; PLD, phospholipase D.
Corresponding author: phone: +7 (913) 218-47-06.
Rights and permissions
About this article
Cite this article
Merkulyeva, Y.A., Shcherbakov, D.N., Sharlaeva, E.A. et al. Phospholipases C from the Genus Bacillus: Biological Role, Properties, and Fields of Application. Russ J Bioorg Chem 47, 653–659 (2021). https://doi.org/10.1134/S1068162021030134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021030134