Abstract
As a rule, coronavirus infections are mild in healthy adults and do not require special approaches to treatment. However, highly pathogenic strains, particularly the recently isolated SARS-CoV2, which causes COVID-19 infection, in about 15% of cases lead to severe complications, including acute respiratory distress syndrome, which causes high patient mortality. In addition, a common complication of COVID-19 is the development of pulmonary fibrosis. Why is the novel coronavirus so pathogenic? What new treatments can be proposed to speed up the recovery and subsequent rehabilitation of the organism? In 2020, over 34 000 scientific articles were published on the structure, distribution, pathogenesis, and possible approaches to the treatment of infection caused by the novel SARS-CoV2 coronavirus. However, there are still no definitive answers to these questions, while the number of the diseased is increasing daily. One of the comprehensive approaches to the treatment of the consequences of the infection is the use of multipotent human mesenchymal stromal cells and products of their secretion (secretome). Acting at several stages of the development of the infection, the components of the secretome can suppress the interaction of the virus with endothelial cells, regulate inflammation, and stimulate lung tissue regeneration, preventing the development of fibrosis. The results of basic and clinical research on this topic are summarized, including our own experimental data, indicating that cell therapy approaches can be successfully applied to treat patients with COVID-19.
Similar content being viewed by others
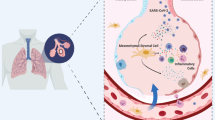
Coronaviruses are spherical viral particles, the morphological features of which include outgrowths covering the envelope, which resemble the solar corona. The outgrowths consist of a highly glycosylated protein, called the S-glycoprotein, or spike. It is the S-glycoprotein that determines the attachment of the virus and its penetration into the target cell. According to the data obtained on cultured cells, the receptor for the spike proteins of both SARS-CoV and SARS-CoV-2 can be angiotensin-converting enzyme 2 (ACE2) [1]. This protein has been found on the surface of cells of the heart, kidneys, adipose tissue, blood vessel walls, brain, testicles, and gastrointestinal tract. Proceeding from the tissue distribution of ACE2, a map of infection with the novel coronavirus SARS-CoV-2 was constructed, according to which lung cells, namely type-II alveolocytes, as well as epithelial cells of the small intestine and cells of the blood vessel wall, including endothelium and pericytes, are at the greatest risk of infection.
Indeed, using electron microscopy, SARS-CoV-2 particles were detected in these cells. Based on the data obtained, a new approach to the treatment of COVID-19 was proposed, which implies blocking of the interaction of ACE2 and the spike protein. However, the clinical efficacy of this approach remains unexplored since the study based on a soluble form of ACE2 was withdrawn before results were obtained. A more detailed study of the interaction of SARS-CoV-2 with cells carrying ACE2 on their surface showed that the presence of this protein does not in itself determine the infection of a cell with the new coronavirus. To bind with the receptors and initiate the fusion of the viral particle with the cell membrane, the spike protein must be activated by proteolytic cleavage at two sites [2]. Thus, the activation of spike proteins of coronaviruses on the cell membrane can be mediated by transmembrane protein serine protease 2 (TMPRSS2). Hence, the efficiency of binding and penetration of viruses into cells that simultaneously carry the ACE2 receptor and the TMPRSS2 protease is much higher compared to cells expressing only the receptor. The efficiency of the cleavage of the spike protein by cellular enzymes is determined, in particular, by its amino acid sequence, which may differ slightly in different strains of the coronavirus and contain sites of recognition by different proteases, including furin, plasmin, kallikrein, cathepsins B and L, and others.
SARS-CoV-2 was found to be able to enter target cells by binding to other membrane proteins as well, including CD147, also known as basigin or matrix metalloprotease inducer [3]. This receptor is present in almost all tissues and organs, where it is located mainly in the endothelium and epithelial lining of the respiratory tract and small intestine, in “immature” (poorly differentiated) cells, as well as in cells of many types of tumors. The tissue distribution of CD147 indicates that it can cause the penetration of SARS-CoV-2 into many tissues and mediate multiple organ failure under severe forms of COVID-19. Proceeding from these data, it was proposed to use a drug that blocks the interaction of CD147 with the spike protein. A clinical study of the efficacy of meplazumab, based on blocking CD147 monoclonal antibodies, showed that its use significantly reduces the severity of the course of the disease and accelerates the elimination of the virus from the body [4].
Thus, the pathway or pathways of cell infection depend(s) on the presence of receptors on its membrane that can bind the spike protein and on the efficiency of its proteolytic activation, which is determined by the availability of the proteases required for this. The enzymes that activate the spike protein can become targets for therapy, and analysis of their content and activity in tissues and blood serum can be used to predict the course of infection.
Vascular wall cells as targets of the new coronavirus. Although in the first months of the pandemic the development of pneumonia was considered one of the initial stages of the novel coronavirus infection, a more detailed analysis showed that damage to the lungs, as well as to other organs, is more likely a consequence of damage to blood vessels [5]. Most likely, the infection of the cells of the vascular wall with SARS-CoV-2 plays the central role in the pathogenesis of COVID-19. For example, throughout the world, patients with COVID-19 have been registered who died of cardiovascular events such as stroke and heart attack, but without any signs of damage to the respiratory system. The endothelium of the vascular wall carries viral receptors, ACE2, CD147, as well as proteases that mediate the cleavage of the spike protein. Infection of endothelial cells is accompanied by the infiltration of the vascular wall by immune cells and the disruption of the integrity of the endothelial monolayer, which can contribute to the formation of blood clots and, as a consequence, multiple organ failure [6].
Earlier, our works and works by other authors established that the wall of blood vessels serves as a special environment for the so-called multipotent mesenchymal stromal cells (MSCs), which are involved in maintaining the cellular composition of tissues and their recovery after damage. Thus, MSCs are required for maintaining the viability of the endothelium and stabilizing blood vessels. In addition, they are able to control the activity of cells of innate and adaptive immunity, including neutrophils, macrophages, and various subpopulations of T cells. Analysis of the atlas of human tissues, compiled on the basis of the RNA content in single cells, showed that MSCs of the heart and skin also have a high content of receptors to SARS-CoV-2 and, consequently, like the endothelium, can become targets of the new coronavirus [7]. We can assume that it is damage to MSCs that initiates the progression of COVID-19 according to an unfavorable scenario. However, the question remains, why this happens only to some patients infected with the new coronavirus. Perhaps the explanation is that the severity of the course of COVID-19 depends on age and the presence of comorbidities, including diabetes and obesity. Note that these conditions, like many other chronic diseases, are associated with dysfunctions of MSCs [8].
Thus, the loss or dysfunction of MSCs due to aging and the development of comorbid diseases in combination with damage by SARS-CoV-2 can contribute to thromboses of the microvascular bed, as well as the development of an excessive immune response, which is expressed in the overproduction of proinflammatory cytokines (the so-called cytokine storm).
The effectiveness of MSC secretion products for the treatment of patients with COVID-19. At present, the tactics for treating severe cases of COVID-19 and its complications remain supportive, aimed at reducing the damage caused by artificial lung ventilation and stimulating endogenous recovery processes. Clinical studies of the effectiveness of several approaches are being conducted, including transfusion of inactivated convalescent plasma; a combination of known antiviral drugs; and the use of anticoagulants, anti-inflammatory drugs, a soluble form of ACE2, and monoclonal antibodies that inhibit interleukin-6 (tocilizumab, etc.).
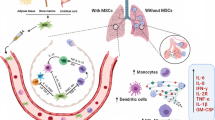
Considering the observations indicating damage to MSCs due to infection with SARS-CoV-2, it can be assumed that an effective approach to treating patients with COVID-19 will be cell therapy using allogeneic MSCs from bone marrow, adipose, or other tissues, which over the last two decades has been viewed as a promising approach for suppressing an excessive immune response and stimulating tissue regeneration. Indeed, 25 ongoing clinical trials are testing MSCs for the treatment of COVID-19, including acute respiratory distress syndrome (ARDS) associated with COVID-19, in various countries (China, the United States, Jordan, Britain, Denmark, and France). Phase 1 and 2 clinical trials have shown the safety of MSC infusions for patients with acute or chronic respiratory distress syndrome [9–12]. More than a dozen clinical cases have demonstrated that treatment with MSCs improves the functioning of the airways; reduces hemodynamic and multiple organ failure; decreases pulmonary and systemic inflammation, apoptosis, and pulmonary edema; and accelerates tissue repair [13, 14].
Many studies have shown that the different effects of MSCs are due to the components of the secretome—a wide range of soluble biologically active molecules: cytokines, growth factors, enzymes, and factors that regulate inflammation [8, 15]. Our earlier proteomic analysis showed that the secretome contains proteins that are involved in maintaining balance between the activity of proteases and their inhibitors, which means that they can prevent both the proteolytic activation of the viral spike protein and the excessive activity of proteases in the tissues affected by the virus in general. Suppression of protease activity can prevent tissue damage and destruction caused by the activity of innate immune cells. The pronounced activity of enzyme systems triggers the inflammatory process and predisposes one to a greater risk of bacterial infections, which in the lungs, for example, serves as a sign of pathologies such as fibrosis and emphysema [16]. MSC secretome also includes factors involved in the antibacterial response [17]. The antiprotease, antimicrobial, and anti-inflammatory effects of MSC secretome make it possible to use it for the treatment of various pulmonary diseases, including pneumonia developing in patients with COVID-19 [18, 19].
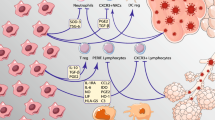
Earlier, we and other researchers showed that components of the MSC secretome suppress the immune response in vivo and in vitro [19–22]. They are able to activate immune cells, stimulate their proliferation, and enhance the secretion of pro- and anti-inflammatory cytokines. The specific pattern of secreted cytokines stimulates the formation of immunosuppressive T-regulatory cells and M2 macrophages [19, 21, 23, 24] and suppresses the activity of other T cells [25]. The most important role in the effects of MSCs is played by cell-secreted anti-inflammatory cytokine IL-10, including during lung tissue regeneration [19, 23]. The MSC secretome also contains growth factors and microRNAs, which increase the viability of endothelial cells and prevent the development of endothelial dysfunction leading to the development of cardiovascular complications in patients with COVID-19.
The ability of MSC secretome to suppress excessive immune response and increase endothelial viability can be enhanced by cultivating cells under hypoxic conditions, in the presence of interferon gamma (IFNγ) or platelet-derived growth factor (PDGF). We have shown that such effects lead to an increase in the content of mediators of the anti-inflammatory and regenerative effects of MSCs, including hepatocyte growth factor (HGF) and growth factor derived from pigment epithelium cells (PEDF), as well as specific microRNAs (miR-223, miR-146b, miR-126, and miR-199a). For example, miR-223 induces the polarization of macrophages into the M2 type, while the expression of miR-146b leads to a significant decrease in the production of the most important proinflammatory cytokines and chemokines, such as IL-6, which play a key role in the development of ARDS [21, 26]. Hypoxia increases the expression of miR-126, which mediates the protective effect of MSCs with respect to vascular integrity and cell differentiation [27]. PDGF plays an important role in lung tissue repair [28]. We showed previously that stimulation of human adipose tissue MSCs with PDGF led to an increase in the immunosuppressive and regenerative properties of their secretome at the expense of stimulating the formation of anti-inflammatory, so-called regulatory T-lymphocytes, and activating the growth of blood vessels [21]. The cultivation of MSCs in the presence of proinflammatory cytokines TNF-α or IFNγ stimulates the production by cells of factors that can induce the polarization of anti-inflammatory M2 macrophages through an increased level of immunosuppressive proteins [29].
Note also that the MSC secretome contains peptidases proposed as coreceptors for SARS-CoV-2. According to our data, supported by the results of other researchers, MSCs express components of the local renin-angiotensin system (RAS), including angiotensin, an angiotensin-converting enzyme, a renin receptor, and receptors for angiotensin peptides [30]. In addition, the MSC secretome is enriched with angiotensinase C, an enzyme that, together with ACE2, participates in the generation of the Ang 1-7 peptide [30]. Thus, the MSC secretome can, when administered, replenish the amount of Ang1-7, preventing cardiac events, in particular, tachycardia caused by the occupation of ACE2 by the SARS-CoV-2 virus. In addition, MSC secretome contains various endopeptidases, including DPP4, which presumably play the role of coreceptors for ACE2.
Thus, MSCs and their secretion products may prove to be an effective therapy for patients with COVID-19 due to their ability to suppress acute inflammation and prevent the development of thrombosis and cytokine storms, which are the main causes of the severe course of the noval coronavirus infection [31–34].
The MSC secretome for preventing and treating pulmonary fibrosis. Chronic or prolonged inflammation often leads to the development of fibrosis—the replacement of functionally active tissue with a connective tissue matrix. For example, pneumonias of viral etiology, which are characterized by a pronounced immune response, often lead to the development of fibrotic complications, deterioration of respiratory function, and severe disability of patients. The main source of excess matrix accumulation is myofibroblasts; therefore, an increase in the number of these cells in the tissue becomes a central event in the development of fibrosis. Thus, suppressing excessive inflammation, as well as preventing the appearance of myofibroblasts in the tissue, can halt the development of fibrosis. However, there are currently no effective treatments for pulmonary fibrosis.
Evidence has been accumulated that MSC secretome can prevent and even reverse pulmonary fibrosis. Most of the results indicate the leading role of products secreted by MSCs in the therapeutic effect of these cells [35]. MSCs or bioactive products secreted by them into the culture medium suppress the development of fibrosis in various in vivo models, such as renal failure, liver fibrosis, and idiopathic pulmonary fibrosis [35–39]. However, the mechanisms of these effects are understudied. Analysis of the MSC secretome revealed the presence of enzymes involved in extracellular matrix remodeling, including matrix metalloproteinases (MMP2 and MMP9) and their inhibitors (TIMP-1 and TIMP-2), as well as fibrosis-related cytokines (IL-10 and prostaglandin-E2), vascular endothelial growth factors (VEGF), and HGF, which contribute to the antifibrotic action of the MSC secretome [17, 37, 40]. Earlier, in in vitro models, we identified specific microRNAs (miR-21, -29, -129) that can mediate the antifibrotic effect of the MSC secretome [41]. Similar data were obtained in other studies [37, 42].
Not only experimental but also clinical data indicate that MSCs are effective for preventing tissue fibrosis [36, 39, 44] due to the immunosuppressive effects, as well as the ability to modulate the profibrotic microenvironment. The data of preclinical studies in this area confirm the effectiveness of the use of MSCs at the initial stage of treatment in the inflammatory phase [39]. Along with this, MSCs have proved their effectiveness in the treatment of severe pulmonary fibrosis, including in comparison with pirfenidone [45]. Thus, a randomized, open-label, and placebo-controlled phase I/IIA study, conducted in Russia, demonstrated the safety, good tolerability, and a number of positive effects of MSCs obtained from bone marrow in patients with rapidly progressing idiopathic pulmonary fibrosis [44, clinical study NCT02594839]. Several early phase clinical trials are currently underway to study autologous or allogeneic MSCs isolated from adipose tissue, the placenta, or the umbilical cord for the treatment of pulmonary fibrosis (www.clinicaltrials.gov). Most of them have confirmed the safety of this approach, but its effectiveness has yet to be proved.
Note that, for the successful introduction of approaches based on the use of products with MSC secretome components into clinical practice, it is necessary to solve a number of problems associated with the standardization of a new class of drugs and the development of strategies for their noninvasive administration, for example, using inhalation [18, 46]. The recently launched pilot clinical trials of the use of the vesicular fraction of MSC secretome for the treatment of severe coronavirus pneumonia (NCT04276987), as well as for determining its inhalation tolerance in healthy volunteers (NCT04313647), are expected to confirm the potential safety and efficacy of this approach.
* * *
Cell therapy and the use of MSC secretome-based products may prove to be very productive in the treatment of critically ill patients with COVID-19 and in combating the complications of coronavirus infection. The first successful results of cell therapy in critically ill patients with coronavirus infection have been obtained: injections of donor MSCs have helped to reduce significantly the level of inflammation in these patients and have led to an improvement in the clinical and radiological picture. The extent to which the injected cells themselves were involved in repairing damaged lungs remains unclear. Undoubtedly, paracrine effects have contributed much to the stimulation of regenerative processes. The accumulated data are still preliminary, but they have encouraged and motivated other researchers to search for and develop optimal protocols for cell therapy. Since cell therapy is associated with some serious risks for the patient and requires a thorough study of the biosafety and efficacy, promising approaches should also include the use of not the cells themselves, but products based on the components of their secretome. According to Russian laws, a specialized licensed production site is required for the production of biomedical cell preparations. The one proposed was created on the basis of the Medical Research and Educational Center of Moscow State University as a part of the Institute for Regenerative Medicine. At present, the process is underway to obtain a license to produce goods for regenerative medicine, which can be used in the treatment of coronavirus infection and its complications.
REFERENCES
W. Li, M. J. Moore, N. Vasilieva, et al., “Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus,” Nature 426 (6965), 450–454 (2003).
S. Belouzard, V. C. Chu, and G. R. Whittaker, “Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites,” Proc. Natl. Acad. Sci. U. S. A. 106 (14), 5871–5876 (2009).
K. Wang, W. Chen, Z. Zhang, et al., “CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells,” Sig. Transduct. Target Ther. 5, 283 (2020).
H. Bian, Z.-H. Zheng, D. We, et al., “Meplazumab treats COVID-19 pneumonia: An open-labelled, concurrent controlled add-on clinical trial,” https://www.medrxiv.org/content/10.1101/2020.03.21.20040691v1. Cited February 1, 2021.
Z. Xu, L. Shi, Y. Wang, et al., “Pathological findings of COVID-19 associated with acute respiratory distress syndrome,” Lancet Resp. Med. 8 (4), 420–422 (2020).
Y. Cao, X. Liu, L. Xiong, and K. Cai, “Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis,” J. Med. Virol. (2020). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7228215/.
F. Qi, S. Qian, S. Zhang, and Z. Zhang, “Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses,” Biochem. Biophys. Res. Comms. 526 (1), 135–140 (2020).
A. Yu. Efimenko, T. N. Kochegura, Zh. A. Akopyan, and Ye. V. Parfyonova, “Autologous stem cell therapy: How aging and chronic diseases affect stem and progenitor cells,” BioRes. Open Access 4 (1), 26–38 (2015).
D. J. Weiss, R. Casaburi, R. Flannery, et al., “A placebo-controlled, randomized trial of mesenchymal stem cells in COPD,” Chest. 143 (6), 1590–1598 (2013).
Y. S. Chang, S. Y. Ahn, H. S. Yoo, et al., “Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial,” J. Pediatrics 164 (5), 966–972 (2014).
G. Zheng, L. Huang, H. Tong, et al., “Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study,” Respir. Res. 15 (1), 39 (2014).
M. A. Matthay, C. S. Calfee, H. Zhuo, et al., “Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial,” Lancet Respir. Med. 7 (2), 154–162 (2019).
S. Atluri, L. Manchikanti, and J. A. Hirsch, “Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: The case for compassionate use,” Pain Physician. 23 (2), E71–E83 (2020).
A. Golchin, E. Seyedjafari, and A. Ardeshirylajimi, “Mesenchymal stem cell therapy for COVID-19: Present or future,” Stem Cell Rev. Rep. 16 (3), 427–433 (2020).
S. Maacha, H. Sidahmed, S. Jacob, et al., “Paracrine mechanisms of mesenchymal stromal cells in angiogenesis,” Stem Cells Int. (2020). https://doi.org/10.1155/2020/4356359
C. Taggart, M. A. Mall, G. Lalmanach, et al., “Protean proteases: At the cutting edge of lung diseases,” Eur. Respir. J. 49 (2), 1501200 (2017).
N. Kalinina, D. Kharlampieva, M. Loguinova, et al., “Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes,” Stem Cell Res. Ther. 6 (221) (2015).
E. Bari, I. Ferrarotti, M. L. Torre, et al., “Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through ‘pharmaceuticalization’ for the best formulation,” J. Controlled Release 309, 11–24 (2019).
C. Lo Sicco, D. Reverberi, C. Balbi, et al., “Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization,” Stem Cells Transl. Med. 6 (3), 1018–1028 (2017).
J. S. Heo, Y. Choi, and H. O. Kim, “Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes,” Stem Cells Int. (2019). https://doi.org/10.1155/2019/7921760
T. Lopatina, C. Grange, V. Fonsato, et al., “Extracellular vesicles from human liver stem cells inhibit tumor angiogenesis,” Int. J. Cancer. 144 (2), 322–333 (2019).
F. Figliolini, A. Ranghino, C. Grange, et al., “Extracellular vesicles from adipose stem cells prevent muscle damage and inflammation in a mouse model of hind limb ischemia: Role of neuregulin-1,” Arterioscler Thromb Vasc. Biol. 40 (1), 239–254 (2020).
A. Eirin, X. Y. Zhu, A. S. Puranik, et al., “Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation,” Kidney Int. 92 (1), 114–124 (2017).
H. Zhao, Q. Shang, Z. Pan, et al., “Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and Beijing in white adipose tissue,” Diabetes 67 (2), 235–247 (2018).
A. Farinazzo, S. Angiari, E. Turano, et al., “Nanovesicles from adipose-derived mesenchymal stem cells inhibit T lymphocyte trafficking and ameliorate chronic experimental autoimmune encephalomyelitis,” Sci. Rep. 8 (1), 7473 (2018).
E. Fan, D. Brodie, and A. S. Slutsky, “Acute respiratory distress syndrome,” JAMA 319 (7), 698 (2018).
D. A. Chistiakov, A. N. Orekhov, and Y. V. Bobryshev, “The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease,” J. Mol. Cell. Cardiol. 97, 47–55 (2016).
L. S. Snyder, M. I. Hertz, M. S. Peterson, et al., “Acute lung injury: Pathogenesis of intraalveolar fibrosis,” J. Clin. Investig. 88 (2), 9663–9673 (1991).
J. An, Q. Li, D. Bhang, et al., “TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis,” Sci. Rep. 10 (1), 2115 (2020).
V. Y. Sysoeva, L. V. Ageeva, P. A. Tyurin-Kuzmin, et al., “Local angiotensin II promotes adipogenic differentiation of human adipose tissue mesenchymal stem cells through type 2 angiotensin receptor,” Stem Cell Res. 25, 115–122 (2017).
Z. Leng, R. Zhu, W. Hou, et al., “Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia,” Aging Dis. 11 (2), 216–228 (2020).
E. N. Worthington and J. S. Hagood, “Therapeutic use of extracellular vesicles for acute and chronic lung disease,” Int. J. Mol. Sci. 21 (7), 2318 (2020).
A. Gowen, F. Shahjin, S. Chand, et al., “Mesenchymal stem cell-derived extracellular vesicles: Challenges in clinical applications,” Front Cell Dev. Biol. 8, 149 (2020).
S. M. Metcalfe, “Mesenchymal stem cells and management of COVID-19 pneumonia,” Med. Drug Discov. 5, 100019 (2020).
A. Rathinasabapathy, E. Bruce, A. Espejo, et al., “Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis,” British J. Pharmacol. 173 (19), 2859–2879 (2016).
B. Usunier, M. Benderitter, R. Tamarat, and A. Chapel, “Management of fibrosis: The mesenchymal stromal cells breakthrough,” Stem Cells Int. 2014 (340257), 1–26 (2014).
S. Samad, K. M. Akram, N. R. Forsyth, and M. A. Spiteri, “Mesenchymal stem cell conditioned media (MSCCM) suppress Wnt-3a and TGF-β1—induced myofibroblastic differentiation,” J. Stem Cells Res., Rev. Rep. 1 (3), 1015 (2014).
N. Srour and B. Thébaud, “Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: A systematic review,” Stem Cells Transl. Med. 4 (12), 1500–1510 (2015).
P. C. Dinh, D. Paudel, H. Brochu, et al., “Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis,” Nature Commun. 11 (1),1064 (2020).
E. F. Cahill, H. Kennell, F. Carty, et al., “Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis,” Stem Cells Transl. Med. 5 (10), 1307–1318 (2016).
N. Basalova, G. Sagaradze, M. Arbatskiy, et al., “Secretome of mesenchymal stromal cells prevents myofibroblasts differentiation by transferring fibrosis-associated microRNAs within extracellular vesicles,” Cells 9, 1272 (2020).
S. Fang, C. Xu, Y. Zhang, et al., “Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing,” Stem Cells Transl. Med. 5 (10), 1425–1439 (2016).
E. El Agha, R. Kramann, R. K. Schneider, et al., “Mesenchymal stem cells in fibrotic disease,” Cell Stem Cell. 21 (2), 166–177 (2017).
A. Averyanov, I. Koroleva, M. Konoplyannikov, et al., “First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline,” Stem Cells Transl. Med. 9, 6–16 (2020).
M. Reddy, L. Fonseca, S. Gowda, et al., “Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: Comparison with pirfenidone,” Int. J. Stem Cells. 9 (2), 192–206 (2016).
G. Sagaradze, O. Grigorieva, P. Nimiritsky, et al., “Conditioned medium from human mesenchymal stromal cells: Towards the clinical translation,” Int. J. Mol. Sci. 20 (7), 1656 (2019).
Funding
Studies on the effectiveness of MSC secretome for the prevention of pulmonary fibrosis were supported by the Russian Foundation for Basic Research, project no. 20-04-60487.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Alekseev
Anastasiya Yur’evna Efimenko, Cand. Sci. (Med.), is Head of the Tissue Reparation and Regeneration Laboratory of the Institute for Regenerative Medicine at the Medical Research and Educational Center of Moscow State University and an Associate Professor in the Department of Biochemistry and Molecular Medicine of the Faculty of Fundamental Medicine of Moscow State University. Natal’ya Igorevna Kalinina, Cand. Sci. (Biol.), is a Leading Researcher in the Department of Biochemistry and Molecular Medicine of the Faculty of Fundamental Medicine of Moscow State University. Kseniya Andreevna Rubina, Dr. Sci. (Biol.), is Head of the Laboratory of Morphogenesis and Tissue Reparation of the Faculty of Fundamental Medicine of Moscow State University. Ekaterina Vladimirovna Semina, Cand. Sci. (Biol.), is a Leading Researcher of the Laboratory of Molecular Endocrinology at the Research Institute of Experimental Cardiology of the National Medical Research Center of Cardiology of the Ministry of Health of Russia and a Senior Researcher of the Research Laboratory of Gene and Cell Technologies of the Faculty of Fundamental Medicine of Moscow State University. Veronika Yur’evna Sysoeva, Cand. Sci. (Biol.), is a Senior Researcher of the Laboratory of Postgenomic Technologies in Medicine of the Faculty of Fundamental Medicine of Moscow State University. Zhanna Alekseevna Akopyan, Cand. Sci. (Med.), is a Leading Researcher of the Institute for Regenerative Medicine at the Medical Research and Educational Center of Moscow State University and Deputy Dean of the Faculty of Fundamental Medicine of Moscow State University. Academician Vsevolod Arsen’evich Tkachuk, Dr. Sci. (Biol.), is Dean of the Faculty of Fundamental Medicine of Moscow State University and Director of the Institute for Regenerative Medicine of the Medical Research and Educational Center of Moscow State University.
Rights and permissions
About this article
Cite this article
Efimenko, A.Y., Kalinina, N.I., Rubina, K.A. et al. Secretome of Multipotent Mesenchymal Stromal Cells as a Promising Treatment and for Rehabilitation of Patients with the Novel Coronaviral Infection. Her. Russ. Acad. Sci. 91, 170–175 (2021). https://doi.org/10.1134/S101933162102012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S101933162102012X