Abstract
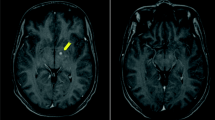
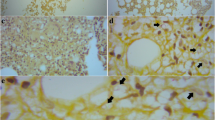
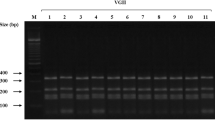
Members of the Cryptococcus gattii species complex are notorious causes of cryptococcosis as they often cause severe, life-threatening infections. Here we describe a case of a severe disseminated C. deuterogattii infection in a previously healthy patient who was initially treated with amphotericin B, 5-fluorocytosine and fluconazole, which led to a good neurological response, but the infection in the lungs remained unaltered and was not completely resolved until switching the antifungal therapy to isavuconazole. The infection was likely acquired during a one-month stay at the Azores Islands, Portugal. Environmental sampling did not yield any cryptococcal isolate; therefore, the source of this apparent autochthonous case could not be determined. Molecular typing showed that the cultured C. deuterogattii isolates were closely related to the Vancouver Island outbreak-genotype.





Similar content being viewed by others
References
Springer DJ, Chaturvedi V. Projecting global occurrence of Cryptococcus gattii. Emerg Infect Dis. 2010;16(1):14–20. https://doi.org/10.3201/eid1601.090369.
Herkert PF, Hagen F, Pinheiro RL, Muro MD, Meis JF, Queiroz-Telles F. Ecoepidemiology of Cryptococcus gattii in developing countries. J Fungi (Basel). 2017;3(4):62. https://doi.org/10.3390/jof3040062.
Galanis E, MacDougall L, Kidd S, Morshed M. British Columbia Cryptococcus gattii working group epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 2010;16(2):251–7. https://doi.org/10.3201/eid1602.090900.
Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A. 2004;101(49):17258–63. https://doi.org/10.1073/pnas.0402981101.
Smith RM, Mba-Jonas A, Tourdjman M, Schimek T, DeBess E, Marsden-Haug N, Harris JR. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS ONE. 2014;9(2):e88875. https://doi.org/10.1371/journal.pone.0088875.
Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. https://doi.org/10.1016/j.fgb.2015.02.009.
Hagen F, Lumbsch HT, Arsic Arsenijevic V, Badali H, Bertout S, Billmyre RB, et al. 2017 Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus genus. mSphere. 2(4): 00238–17. Doi: https://doi.org/10.1128/mSphere.00238-17
Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. 2017 The case for adopting the "species complex" nomenclature for the etiologic agents of cryptococcosis. mSphere. 2(1): 00357–16. doi: https://doi.org/10.1128/mSphere.00357-16.
Cogliati M, Desnos-Ollivier M, McCormick-Smith I, Rickerts V, Ferreira-Paim K, Meyer W, et al. Genotypes and population genetics of Cryptococcus neoformans and Cryptococcus gattii species complexes in Europe and the Mediterranean area. Fungal Genet Biol. 2019;129:16–29. https://doi.org/10.1016/j.fgb.2019.04.001.
Cogliati M, D’Amicis R, Zani A, Montagna MT, Caggiano G, De Giglio O, et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016. https://doi.org/10.1093/femsyr/fow086.
Hagen F, Colom MF, Swinne D, Tintelnot K, Iatta R, Montagna MT, et al. Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis. 2012;18(10):1618–24. https://doi.org/10.3201/eid1810.120068.
Smith IM, Stephan C, Hogardt M, Klawe C, Tintelnot K, Rickerts V. Cryptococcosis due to Cryptococcus gattii in Germany from 2004–2013. Int J Med Microbiol. 2015;305(7):719–23. https://doi.org/10.1016/j.ijmm.2015.08.023.
Cárdenes M, Angel-Moreno A, Fieschi C, Sologuren I, Colino E, Molinés A, et al. Oesophageal squamous cell carcinoma in a young adult with IL-12R beta 1 deficiency. J Med Genet. 2010;47(9):635–7. https://doi.org/10.1136/jmg.2009.071910.
Esteve-Solé A, Sologuren I, Martínez-Saavedra MT, Deyà-Martínez À, Oleaga-Quintas C, Martinez-Barricarte R, et al. Laboratory evaluation of the IFN-γ circuit for the molecular diagnosis of Mendelian susceptibility to mycobacterial disease. Crit Rev Clin Lab Sci. 2018;55(3):184–204. https://doi.org/10.1080/10408363.2018.1444580.
Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernández-Pérez L, Chapgier A, et al. Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet. 2011;20(8):1509–23. https://doi.org/10.1093/hmg/ddr029.
Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti–GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190(8):3959–66.
Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, et al. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods. 2014;402(1–2):57–70. https://doi.org/10.1016/j.jim.2013.11.011.
Tomazin R, Matos T, Meis JF, Hagen F. Molecular characterization and antifungal susceptibility testing of sequentially obtained clinical Cryptococcus deneoformans and Cryptococcus neoformans isolates from Ljubljana. Slovenia Mycopathologia. 2018;183(2):371–80. https://doi.org/10.1007/s11046-017-0214-9.
Kinne J, Joseph M, Wernery U, Nogradi N, Hagen F. Disseminated Cryptococcus deuterogattii (AFLP6/VGII) infection in an Arabian horse from Dubai. United Arab Emirates Rev Iberoam Micol. 2017;34(4):229–32. https://doi.org/10.1016/j.riam.2017.02.007.
Bauer M, Wickenhauser C, Haak A, Pazaitis N, Siebolts U, Mawrin C, et al. Case report: a fatal case of cryptococcosis in an immunocompetent patient due to Cryptococcus deuterogattii (AFLP6/VGII). JMM Case Rep. 2018;5(10):e005168. https://doi.org/10.1099/jmmcr.0.005168.
Georgi A, Schneemann M, Tintelnot K, Calligaris-Maibach RC, Meyer S, Weber R, Bosshard PP. Cryptococcus gattii meningoencephalitis in an immunocompetent person 13 months after exposure. Infection. 2009;37(4):370–3. https://doi.org/10.1007/s15010-008-8211-z.
Hagen F, van Assen S, Luijckx GJ, Boekhout T, Kampinga GA. Activated dormant Cryptococcus gattii infection in a Dutch tourist who visited Vancouver Island (Canada): a molecular epidemiological approach. Med Mycol. 2010;48(3):528–31. https://doi.org/10.3109/13693780903300319.
Colom MF, Hagen F, Gonzalez A, Mellado A, Morera N, Linares C, et al. Ceratonia siliqua (carob) trees as natural habitat and source of infection by Cryptococcus gattii in the Mediterranean environment. Med Mycol. 2012;50(1):67–73. https://doi.org/10.3109/13693786.2011.574239.
Chowdhary A, Randhawa HS, Boekhout T, Hagen F, Klaassen CH, Meis JF. Temperate climate niche for Cryptococcus gattii in Northern Europe. Emerg Infect Dis. 2012;18(1):172–4. https://doi.org/10.3201/eid1801.111190.
Hoang L, Philips P, Galanis E. Cryptococcus gattii: a review of the epidemiology, clinical presentation, diagnosis and management of this endemic yeast in the Pacific Northwest. Clin Microbiol Lett. 2011;33(24):187–95. https://doi.org/10.1016/j.clinmicnews.2011.11.003.
Ulett KB, Cockburn JW, Jeffree R, Woods ML. Cerebral cryptococcoma mimicking glioblastoma. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2016-218824.
Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, et al. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis. 2012;55(6):789–98. https://doi.org/10.1093/cid/cis529.
Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31(2):499–508. https://doi.org/10.1086/31399229.
World Health Organization (WHO). Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva, World Health Organization, 2018.
Bloch KC, Bailin SS. Update on fungal infections of the central nervous system: emerging pathogens and emerging diagnostics. Curr Opin Infect Dis. 2019;32(3):277–84. https://doi.org/10.1097/QCO.0000000000000541.
Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48(7):856–62. https://doi.org/10.1086/597262.
Bruner KT, Franco-Paredes C, Henao-Martínez AF, Steele GM, Chastain DB. Cryptococcus gattii complex infections in HIV-infected patients Southeastern United States. Emerg Infect Dis. 2018;24(11):1998–2002. https://doi.org/10.3201/eid2411.180787.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. https://doi.org/10.1086/649858.
Chen SC, Korman TM, Slavin MA, Marriott D, Byth K, Bak N, et al. Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin Infect Dis. 2013;57(4):543–51. https://doi.org/10.1093/cid/cit341.
Lawrence DS, Boyer-Chammard T, Jarvis JN. Emerging concepts in HIV-associated cyptococcal meningitis. Curr Opin Infect Dis. 2019;32(1):16–23. https://doi.org/10.1097/QCO.0000000000000514.
Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Guinea J, Hagen F, Meis JF, et al. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27–A3 broth microdilution method. Antimicrob Agents Chemother. 2015;59(1):666–8. https://doi.org/10.1128/AAC.04055-14.
Thompson GR, Rendon A, Ribeiro dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016;63:356–62. https://doi.org/10.1093/cid/ciw305.
Garcia-Vidal C. Current therapeutic options in invasive mycosis and potential therapeutic role of isavuconazole. Rev Iberoam Micol. 2018;35(4):192–7. https://doi.org/10.1016/j.riam.2018.07.003.
Ellsworth M, Ostrosky-Zeichner L. Isavuconazole: Mechanism of action, clinical efficacy, and resistance. J Fungi. 2020;6:324. https://doi.org/10.3390/jof6040324.
Galanis E, Hoang L, Kibsey P, Morshed M, Phillips P. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: Lessons learned from British Columbia. Can J Infect Dis Med Microbiol. 2009;20(1):23–8. https://doi.org/10.1155/2009/719659.
Tsujisaki RA, Paniago AM, Lima Júnior MS, Alencar Dde S, Spositto FL, de Nunes MO, et al. First molecular typing of cryptococcemia-causing Cryptococcus in central-west Brazil. Mycopathologia. 2013;176(3–4):267–72. https://doi.org/10.1007/s11046-013-9676-6.
Acknowledgements
The authors thank the patient for consenting to the publication of this case. We are grateful to Ms Isabel Martinez-Hervás Librarian of Severo Ochoa University Hospital for searching literature and to Ms Elisa Parrilla for her assistance in environmental sample collection in Azores Islands. We also want to thank Pablo Flores for his professional review of the English language and Amanda S. Cuétara for her assistance in the design of Fig. 4. Finally, we thank Master Labor SL Company for the donation of the Biosynex CryptoPS kit.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this work.
Ethical Approval
The Ethics Committee of the Severo Ochoa Hospital approved the publication of the case.
Informed Consent
Informed consent was also obtained from the patient for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Patrick CY Woo.
Rights and permissions
About this article
Cite this article
Cuetara, M.S., Jusdado Ruiz-Capillas, J.J., Nuñez-Valentin, M.P. et al. Successful Isavuconazole Salvage Therapy for a Cryptococcus deuterogattii (AFLP6/VGII) Disseminated Infection in a European Immunocompetent Patient. Mycopathologia 186, 507–518 (2021). https://doi.org/10.1007/s11046-021-00566-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00566-w