Abstract
A potential cause of the dissemination of the potato ring rot bacterium Clavibacter sepedonicus (Cs) is the use of automated seed potato cutters. The present study focuses on the question of whether disinfection practices are sufficient to prevent the transmission of Cs from contaminated machine parts to a new tuber lot. The disinfection efficacy was determined by establishing the culturability of Cs that remained after spray application of sodium-p-toluenesulfochloramide solution on clean and fouled specimens of machinery material that had been provided with an imprint of Cs biofilm. Although conventional spraying, with the authorized concentration of sodium-p-toluenesulfochloramide, of inoculated rubber, PVC and lacquered steel led to a substantial decrease of colony forming units, the treatment was insufficient for complete eradication of Cs. The presence of dirt negatively affected the efficacy of the disinfectant.
Similar content being viewed by others
Introduction
Bacterial ring rot (BRR) is a potato disease caused by the Gram-positive bacterium Clavibacter sepedonicus (Cs). The disease is named after its characteristic soft rotting of the vascular tuber ring. Ring rot-infected tubers can carry high numbers of cells (De Boer and McCann 1990). When tubers are cut and squeezed, milky droplets of bacterial slime can arise which sink down upon relief. Other symptoms are curling, wilting and discoloration of the leaves. In many countries including those belonging to the European Union, the pathogen is listed as a quarantine organism. Although Cs, as a hemibiotrophic bacterium, is dependent on its host plants, it is able to survive on contaminated surfaces for years, in particular at relatively low temperature and low humidity (Nelson 1978, 1979, 1980; Nelson and Kozub 1990; Ward et al. 2001). It is therefore important to take precautions to minimise the spread of Cs via contaminated soil, organic matter, storage facilities and equipment.
A potential cause of Cs dissemination is the use of seed potato cutters. Cutting seed potatoes in halves doubles the number of seed pieces, which may be a solution for shortages in supply of seed potato or to reduce costs of planting material. In the Netherlands, seed potato cutting is restricted to the production of consumers’ and starch potatoes. The hygiene protocol prohibits cutting of tubers for seed production and cutting at sites where tubers for seed production are cultivated. The cutting process essentially comprises automatic feeding of individual tubers via shakers and conveyer belts to rotating knives and subsequent collection of the produced tuber halves via conveyer belts into storage boxes. The disk-shaped stainless-steel cutting knives are continuously cleaned by an automatic disinfection system. Immediately after cutting, the tuber halves are slightly powdered with talc, to absorb moisture leaking from wounded tubers.
After the cutting of each tuber lot, the cutting equipment is treated with a spray-application of disinfectant using a handheld sprayer, not a jet cleaner. The applied volume per unit of surface is limited to prevent dripping; the machine parts actually are covered with a haze of a biocide solution. Usually, an incubation period of 15 min precedes cutting of the subsequent tuber lot (Van Tilburg, De Kubbe BV, personal communication).
In spite of the hygiene procedures applied, the slimy surfaces of BRR-affected tuber halves remain a potential source of Cs that can be disseminated via contact with machinery parts and clean tuber halves. The risk of cross contamination of tuber lots is determined by (1) the probability that the cutting machinery catches vital and transmittable Cs from a BRR-affected tuber lot and (2) the probability that this Cs contamination subsequently is transmitted to another tuber lot during its processing in the machine.
The present study focuses on the question of whether the conventional disinfection measures are sufficient to prevent the transmission of Cs from contaminated machine parts to a new tuber lot, i.e. probability number 2. The disinfection efficacy was determined by establishing the culturability of Cs that remained after application of the conventional disinfection treatment on clean and fouled specimens of machinery material that had been provided with an imprint of Cs biofilm.
A Cs-infected potato tuber which travels all the way through an automated potato tuber cutter, ending up in halves in a storage box, meets several surfaces on which it may leave behind spots of Cs biofilm. As judged from their surface area, conveyer belts and the walls that shape the path of travel through the cutting process are the most likely candidates to catch Cs contamination. The type of material presented by these surfaces is one of the factors that may determine the chances of survival of the Cs bacteria attached (Nelson 1978, 1980; Howard et al. 2015). The experimental objects chosen for this study therefore were pieces cut from the original polyvinylchloride conveyer belt that carries the tubers inside the machine in the horizontal plane, pieces cut from the original conveyer belt that carries the tuber halves in vertical direction to the storage box and original pieces of lacquered steel that represent the machine walls and machine rims of the most commonly used potato cutter in the Netherlands. Although the stainless-steel knives also stand high chances to encounter Cs when Cs-infected tubers are processed, this part of the machinery was not taken into account since these disk-shaped devices are continuously cleaned by an automatic disinfection system.
During operation, machine parts may become more or less foul due to deposition of soil, organic material (mainly starch), tuber moisture and talc powder. Dirt is a potential scavenger for the chemical activity of the disinfectant or may eventually affect the viability of Cs in other ways. Therefore, the efficacy of the disinfection procedure with the biocide Halamid was tested on Cs biofilms applied to clean objects as well as on Cs biofilms applied to objects that were provided with a standard layer of potato starch and talc powder. In previous studies, Halamid, a hypochlorite-generating compound (sodium-p-toluenesulfochloramide), was able to disinfect wooden potato crates contaminated with a homogenized potato tuber pulp in 2 min using a jet cleaner (Stevens et al. 2017). It was shown more effective than three other biocides, didecyldimethylammoniumchloride, benzoic acid and peroxysulfate.
Materials and Methods
Disinfection Product
The trade name of the disinfectant used is Halamid-d (81% sodium-p-toluenesulfochloramide). The concentration applied was 1% (w/v), which is the authorised concentration, recommended by the supplier.
Preparation of the Inoculum
The experimental work was performed with Clavibacter sepedonicus (Cs), strain IPO 1873, which is a spontaneous streptomycin resistant mutant of strain NCPB 4053. A suspension of the bacterium (50 μl per plate of circa 108 colony forming units (cfu)/ml) was grown for 6–7 days at 20 °C in small Petri dishes (3 cm in diameter) that were completely filled with Yeast Glucose agar Medium (YGM) from which the convex menisci rose above the rim of each dish. YGM contained 2.0 g/L bacto yeast extract (Difco), 2.5 g/L D-glucose (monohydrate), 0.25 g/L K2HPO4, 0.25 g/L KH2PO4, 0.1 g/L MgSO4·7 H2O, 0.015 g/L MnSO4·H2O, 0.05 g/L NaCl, 0.005 g/L FeSO4·7 H2O, 18 g/L purified agar no. 3 (Oxoid) and additionally 1 mL of a stock solution of streptomycin (final concentration 100 mg/L) and 1 mL of cycloheximide (final concentration 200 mg/L).
Preparation and Inoculation of the Experimental Target Objects for Disinfection
Rectangular polyvinylchloride (PVC) and rubber pieces (2 × 3 cm), cut from original PVC and rubber transport belts, and spray lacquered, steel spare parts (46 x 30 x 6 mm), all kindly provided by Miedema Landbouwwerktuigenfabriek BV (Winsum, the Netherlands), served as experimental target objects. These objects were cleaned, sterilized in 70% ethanol, and subsequently in 96% ethanol, and dried. The efficacy of the disinfection procedure was tested on Cs biofilms applied to clean objects as well as on Cs biofilms applied to objects that were provided with a standard layer of potato starch and talc powder. Therefore, the surface (one side) of the experimental objects was provided either directly with a smear of Cs biofilm or with a smear of Cs biofilm after application of starch and talc powder. In this report, we refer to these two types of experimental objects as ‘clean’ and ‘dirty’ objects, respectively. Starch was applied by spreading over the object surface a 1:1 (w/v) suspension of potato starch (Sigma-Aldrich) in demineralised water (circa 0.4 g of suspension per object). After drying for 20 min, the layer of starch was thinly powdered with talc (Sigma-Aldrich) using a tea strainer. To ensure that Cs biofilms were applied onto all objects in a reproducible way with the same force, the biofilms were transferred to the objects by placing the small Petri dishes onto the objects with the Cs biofilm facing the objects’ surface. Under the pressure of the Petri dish’s mass, this resulted in all cases in a clearly visible print of Cs biofilm.
Disinfection Treatments
The inoculated target objects were treated with a haze of 1% Halamid or water (0% Halamid), applied at circa 10 cm distance with a handheld spray bottle by pushing the button of the vaporizer twice, which resulted in completely and thinly wetted target surfaces. In practice, the disinfectant should be effective within about 15 min before the next seed lot is processed (Van Tilburg, De Kubbe BV, personal communication). Therefore, the objects were incubated at room temperature for 0, 5, 10 or 20 min. The incubations were stopped by submerging the objects separately in 50 ml sterile demineralized water for 1 min. The Cs biofilms were subsequently suspended by rinsing the objects separately in 20 ml sterile neutralizer solution for 30 min using a laboratory shaker. The neutralizer solution, serving as scavenger for disinfectant remnants to protect the bacteria, consisted of 1 g/L L-histidine, 1 g/L L-cysteine and 2 g/L reduced glutathione in water (Tremaroli et al. 2008; Howard et al. 2015). The experiments were performed in 5-fold (experiments A–E).
Sampling, Plating and Colony Counting
Samples (100 μL) of the collected Cs suspensions were plated undiluted and 1000 times diluted in neutralizer solution on agar medium YGM containing 100 mg/L of streptomycin and 100 mg/L of cycloheximide (Van der Wolf and Van Beckhoven 2004). After 1–2 weeks of incubation of the plates at 20–23 °C, the Cs colonies formed were counted. The number of colonies per sample was expressed as log (cfu +1) per cm2 of material.
Colony TaqMan Assay
DNA extracts were prepared by picking slime from individual Cs colonies using a plastic pipette tip, suspending the sample in 50 μL of Milli-Q water and subsequent boiling of the mixtures for 5 min at 95 °C. Two microliters of these DNA preparations were added to a mix of ROXII (0.5x), Premix Ex Taq (1x) from TaKaRa BioInc, forward primer Cs 50-2F (5′-CGGAGCGCGATAGAAGAGGA), reverse primer Cs (5′-GGCAGAGCATCGCTCAGTACC) 133-R (0.3 μM each) and probe FAM-Cs 50-53T (5′-AAGGAAGTCGTCGGATGAAGATGCG) (0.1 μM), to a total volume of 25 μL (Schaad et al. 1999). The real-time PCR amplification was performed using a real-time PCR system ABI7500 (applied Biosystems BV) with the following cycling conditions: initial denaturation for 2 min at 95 °C, then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Following the same protocol, a second colony TaqMan assay was performed on the same DNA-extracts using the NYtor primers described by Vreeburg et al. (2018), i.e. CmsF (5′-TGCTGATAACGTGAT CAA), CmsR (CTGAGCAACGACAAGAAA) and CmsP (5′-ATG GCT CCT CGG TCC TTG AAT GTC).
Statistical Analysis
Analysis of variance was performed on bacterial numbers per agar plate using Genstat (VSN International, 2015. Genstat for Windows 18th Edition. VSN International, Hemel Hempstead, UK. Web page: Genstat.co.uk.). Fisher’s Least Significant Difference was used as post hoc test. In case the number of colonies was uncountable (>>), calculations were based on the assumption that the plates contained 1000 colonies (107–108 cfu per cm2 of material).
Results
In agreement with conventional disinfection practice, the experimental objects were exposed to a haze of 1% (w/v) of the disinfectant Halamid. Special care was taken that the inoculated surfaces were completely covered with disinfectant. Table 1 presents an overview of the number of Cs colonies observed on the plate cultures of all undiluted and 1000 times diluted samples collected from the experimental objects after application of the various Halamid treatments. The identity of the colonies was confirmed by subjecting randomly picked colonies to colony TaqMan PCR, assuming Ct values equal to or below 28 as Cs positive and Ct values above 28 as Cs negative. All colonies checked (164 in total) were Cs positive, except two, both of which exhibited an atypical appearance.
As expected, the undiluted and the 1000 time diluted samples collected from the control objects (i.e. treated with 0% Halamid) all exhibited Cs biofilms grown from uncountable number of colonies with the exception of (most of) the samples that were obtained from the clean lacquered steel objects (Table 1). This observation indicates that attachment of Cs biofilm to clean lacquered steel is less strong than to the other experimental objects, leading to a lower Cs loading on lacquered steel during inoculation or to a partial detachment of bacterial cells during the 1 min of incubation in water before suspension in neutralizing buffer. As estimated from the figures presented by these clean lacquered steel objects, the inoculum printed on the objects contained 108 cfu to 109 cfu per object. Treatment of the clean objects with 1% Halamid for 0 min (i.e. immediate immersion in water and washing the objects in neutralizing solution after application of 1% Halamid) in various cases already resulted in some decrease of bacterial numbers (Table 1). On average, the rubber material contained the highest bacterial numbers after treatment with 1% Halamid, followed by PVC and lacquered steel, respectively. The presence of dirt negatively affected the efficacy of the 1% Halamid treatment (Table 1). Incubation times of 5, 10 and 20 min after application of 1% Halamid led to a substantial decrease of culturable Cs (Table 1). However, none of the treatments consistently led to eradication of Cs in all five experiments A–E, except for the treatment of clean lacquered steel with 1% Halamid combined with an incubation period of 5 min (Table 1).
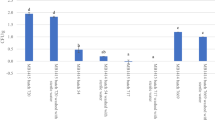
Instead of presenting a countable number of clearly distinguishable colonies, various 1000 times diluted samples which were collected from the objects that were treated with 1% Halamid presented a relatively dense Cs biofilm on the agar plates, grown from uncountable number of colonies (indicated with “>>” in Table 1). To enable statistical data analysis, these Cs biofilms were assumed to represent an estimated number of 1000 colonies per agar plate, which theoretically comes down to 2 × 108 cfu per object. Figure 1 shows the calculated average number of colonies that are based on this assumption. Analysis of variance showed that the material (lacquered steel, PVC and rubber), the absence or presence of dirt (starch/talc) and the incubation time all had a significant effect (F pr.<0.05) on the number of culturable Cs. When calculations were based on the assumption that the uncountable number of colonies represent 10,000 instead of 1000 colonies per plate (2 × 109 cfu per object), analysis of variance showed that all these factors (i.e. type of material, dirt and incubation time) had virtually the same significant effects (P<0.05).
Estimated average number of Clavibacter sepedonicus expressed as log (cfu +1) per cm2 object that developed on agar plates collected from various clean and dirty (starch and talc) objects that were treated with 1% Halamid. Calculations were based on the assumption that the plates which exhibited an uncountable high number of colonies contained 1000 colonies (107–108 cfu per cm2) (N=5). The least significant difference (LSD, P=0.05) estimate is displayed
Discussion
Already for at least 80 years, potato seed cutters have been recognized as a potential source of contamination for bacterial ring rot (Starr 1940; Metzger and Binkley 1940; Dykstra 1941). Starr (1940), for example, showed that the cutting of infected tubers without proper disinfection of the seed cutter increased the infection rate from 23 to 72% as compared to the use of whole seed. By means of field trials, Lane (1949) showed that ring rot can be transmitted via the potato cutter blade by smearing the blade beforehand with a ring rot-infected tuber. From early studies, it also became clear that satisfactory prevention of spread of ring rot via the cutter blade can be accomplished by a continuous flow of hypochlorite containing disinfectant over both sides of the cutting blades during operation (e.g. Lane 1949). As a standard, therefore, potato cutting machines are equipped with spraying devices that provide such continuous treatment of the cutter blades.
It is general practice, however, to disinfect the other parts of the machinery by a low-pressure spray application of disinfectant only in between the cutting of separate seed lots (Van Tilburg, De Kubbe BV, personal communication). The smear of ring rot-infected tubers comprises Cs biofilms that are sticky and are able to become relatively firmly attached to various kinds of material. In addition, it should be noted that bacteria present in microbial biofilms generally exhibit a significantly higher tolerance towards antimicrobials than planktonic cells (Howard et al. 2015). In this study, we therefore investigated the efficacy of this conventional treatment using the ring rot bacterium present in Cs biofilms attached to relevant conveyer belt and lacquered steel materials.
The observation that the type of material of the objects had a significant effect on the number of culturable Cs can be explained by their different capacities to keep Cs biofilm attached to their surface, as has been discussed above with respect to lacquered steel. However, it cannot be excluded that additionally the active component of Halamid solutions is dissipated by the various materials at different rates. The reactive compounds of Halamid solution are sodium-p-toluenesulfochloramide (also named chloramine-T) and its derivative hypochlorite, which is generated from sodium-p-toluenesulfochloramide upon solubilisation of Halamid in water (Seevers and Counsell 1982; Tashtoush et al. 2001). The significant positive effect of dirt (starch/talc) on the survival of Cs strongly indicates that dissipation of the Halamid reactants by non-Cs components like supporting materials plays an important role in the efficacy of the treatments. Interestingly, the decrease of Cs after 1% Halamid application was substantial within the first 5 min of incubation, whereas in the 15-min period thereafter, Cs decrease was almost negligible (Fig. 1). Apparently, the active components of Halamid were dissipated rather quickly, suggesting that at least an increase of Halamid concentration or repeated applications of 1% Halamid is required for complete eradication of Cs. An alternative explanation for this observation is that the remaining Cs cells were shielded from the disinfectant.
In conclusion, mere spray application of the disinfectant Halamid in the authorized concentration on a seed cutter will reduce but not eliminate the risk for transmission of Cs between seed lots. Hygiene protocols advocate the removal of plant material and soil in advance of the disinfection step (Anonymous 2006; Olsen and Nolte 2011). In previous research, a powerful washing to remove dirt and to break up the biofilm present before application of Halamid has been shown effective to disinfect wooden potato crates (Stevens et al. 2017). We therefore expect that a cleaning step before treatment with a biocide will also be effective to disinfect seed cutters.
References
Anonymous (2006) Disinfection procedures in potato production. EPPO Bull 36:463–466
De Boer SH, McCann M (1990) Detection of Corynebacterium sepedonicum in potato cultivars with different propensities to express ring rot symptoms. Am Potato J 67:685–694
Dykstra TP (1941) Results of experiments in control of bacterial ring rot of potatoes in 1940. Am Potato J 18:27–55
Howard RJ, Harding MW, Daniels GC, Mobbs SL, Lisowski SLI, De Boer SH (2015) Efficacy of agricultural disinfectants on biofilms of the bacterial ring rot pathogen, Clavibacter michiganensis subsp. sepedonicus. Can J Plant Pathol 37:273–284
Lane GH (1949) Disinfection of a new stationary-type seed-potato cutter to control the spread of ring rot. Am Potato J 26:379–384
Metzger CH, Binkley AM (1940) Some evidence of the spread of bacterial wilt. Am Potato J 17:198–201
Nelson GA (1978) Survival of Corynebacterium sepedonicum on contaminated surfaces. Am Potato J 55:449–453
Nelson GA (1979) Persistence of Corynebacterium sepedonicum in soil and in buried potato stems. Am Potato J 56:71–77
Nelson GA (1980) Long-term survival of Corynebacterium sepedonicum on contaminated surfaces and in infected potato stems. Am Potato J 57:595–599
Nelson GA, Kozub GC (1990) Survival of Corynebacterium sepedonicum at freezing and at wide fluctuations between freezing and above-freezing temperatures. Am Potato J 67:625–631
Olsen N, Nolte P (2011) Cleaning and disinfecting potato equipment and storage facilities. University of Idaho Extension, CIS, Moscow, p 1180
Schaad NW, Berthier-Schaad Y, Sechler A, Knorr D (1999) Detection of Clavibacter michiganensis subsp. sepedonicus in potato tubers by BIO-PCR and an automated real-time fluorescence detection system. Plant Dis 83:1095–1100
Seevers RH, Counsell RE (1982) Radioiodination techniques for small organic molecules. Chem Rev 82:575–590
Starr GH (1940) Experimental work for the control of ring rot of potatoes. Am Potato J 17:318–322
Stevens LH, Lamers JG, Van der Zouwen P, Mendes O, Van den Berg W, Tjou-Tam-Sin NNA, Jilesen CJTJ, Spoorenberg PM, Van der Wolf J (2017) Chemical eradication of the ring rot bacterium Clavibacter michiganensis subsp. sepedonicus on potato storage crates. Potato Res 60:145–158
Tashtoush BM, Traboulsi AA, Dittert L, Hussain AA (2001) Chloramine-T in radiolabeling techniques. IV. Penta-O-acetyl-N-chloro-N-methylglucamine as an oxidising agent in radiolabeling techniques. Anal Biochem 288:16–21
Tremaroli V, Fedi S, Turner RJ, Ceri H, Zannoni D (2008) Pseudomonas pseudoalcaligenes KF707 upon biofilm formation on a polystyrene surface acquire a strong antibiotic resistance with minor changes in their tolerance to metal cations and metalloid oxyanions. Arch Microbiol 190:29–39
Van der Wolf JM, Van Beckhoven JRCM (2004) Factors affecting survival of Clavibacter michiganensis subsp. sepedonicus in water. J Phytopathol 152:161–168
Vreeburg RAM, Zendman AJW, Pol A, Verheij E, Nas M, Kooman-Gersmann M (2018) Validation of four real-time TaqMan PCRs for the detection of Ralstonia solanacearum and/or Ralstonia pseudosolanacearum and/or Clavibacter michiganensis subsp. sepedonicus in potato tubers using a statistical regression approach. Bull OEPP/EPPO Bull 46:112–121
Ward L, D’Aubin J, De Boer SH (2001) Persistence of Clavibacter michiganensis subsp. sepedonicus in sterilized soil but failure to confirm its survival overwinter in field soil. In: De Boer SH (ed) Plant Pathogenic Bacteria. Kluwer Academic Press, Dordrecht, pp 375–378
Funding
This study was financially supported by the Dutch Ministry of Economic Affairs. The authors thank D. van Tilburg (De Kubbe BV, Biddinghuizen, The Netherlands) and C. Poot (Miedema Landbouwwerktuigenfabriek BV, Winsum, The Netherlands) for providing valuable information about mechanised potato cutting. The authors thank N.N.A. Tjou-Tam-Sin and C.J.T.J. Jilesen (Netherlands Food and Consumer Product Safety Authority, Utrecht, The Netherlands) for the helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stevens, L.H., Tom, J.Y., Mendes, O. et al. Chemical Disinfection of Potato Cutting Machinery to Avoid Dissemination of Clavibacter sepedonicus. Potato Res. 65, 21–29 (2022). https://doi.org/10.1007/s11540-021-09506-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-021-09506-z