Abstract
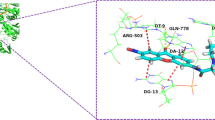
A series of 8,9-substituted Luotonin A analogs were designed, synthesized, and evaluated for antiproliferative activity against four cancer cell lines. The structure–activity relationship study revealed that the in vitro anticancer activity of Luotonin A was significantly improved by the introduction of 8-piperazine group and the 5-deaza modification. Two promising compounds 6a and 7a displayed potent topoisomerase I inhibitory activity. And a rational binding mode of 7a with topoisomerase I–DNA complex was proposed based on the molecular docking study.







Similar content being viewed by others
References
Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. https://doi.org/10.1021/cb300648v
Liang X, Wu Q, Luan S, Yin Z, He C, Yin L, et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur J Med Chem. 2019;171:129–68. https://doi.org/10.1016/j.ejmech.2019.03.034
Li QY, Zu YG, Shi RZ, Yao LP. Review camptothecin: current perspectives. Curr Med Chem. 2006;13:2021–39. https://doi.org/10.2174/092986706777585004
Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M, Centamore A, et al. The long story of camptothecin: from traditional medicine to drugs. Bioorg Med Chem Lett. 2017;27:701–7. https://doi.org/10.1016/j.bmcl.2016.12.085
Yang XY, Zhao HY, Lei H, Yuan B, Mao S, Xin M, et al. Synthesis and biological evaluation of 10-substituted camptothecin derivatives with improved water solubility and activity. ChemMedChem. 2021;16:1000–10. https://doi.org/10.1002/cmdc.202000753
Zi CT, Yang L, Dong FW, Kong QH, Ding ZT, Zhou J, et al. Synthesis and antitumor activity of camptothecin-4β-triazolopodophyllotoxin conjugates. Nat Prod Res. 2020;34:2301–9. https://doi.org/10.1080/14786419.2018.1538223
Song ZL, Yang GZ, Li JC, Liu YQ, Yang CJ, Goto M, et al. Design and synthesis of novel 7-[(N-substituted-thioureidopiperazinyl)-methyl]-camptothecin derivatives as potential cytotoxic agents. Nat Prod Res. 2020;34:2022–9. https://doi.org/10.1080/14786419.2019.1573231
Hong BH, Meng GR, Tan HY, Li JJ, Kong KM, Zhang Q. Synthesis and antitumor activity of pyrano[3,2-i]-fused camptothecin derivatives. Med Chem Res. 2019;28:884–91. https://doi.org/10.1007/s00044-019-02342-4
Wang XH, Yang FH, Zhao CK, Gao L, Li C. Sealed tube promoted coupling of camptothecin and norcantharidin acid ester and their preliminary biological activity evaluation in vitro. Med Chem Res. 2018;27:406–11. https://doi.org/10.1007/s00044-017-2066-8
Zhang XQ, Cao M, Xing J, Liu F, Dong P, Tian X, et al. TQ-B3203, a potent proliferation inhibitor derived from camptothecin. Med Chem Res. 2017;26:3395–406. https://doi.org/10.1007/s00044-017-2032-5
Seiter K. Toxicity of the topoisomerase I inhibitors. Expert Opin Drug Saf. 2005;4:45–53. https://doi.org/10.1517/14740338.4.1.45
Zhang B, Dou Z, Xiong Z, Wang N, He S, Yan X, et al. Design, synthesis and biological research of novel N-phenylbenzamide-4-methylamine acridine derivatives as potential topoisomerase I/II and apoptosis-inducing agents. Bioorg Med Chem Lett. 2019;29:126714–20. https://doi.org/10.1016/j.bmcl.2019.126714
Szafran MJ, Kolodziej M, Skut P, Medapi B, Domagała A, Trojanowski D, et al. Amsacrine derivatives selectively inhibit mycobacterial topoisomerase I (TopA), impair M. smegmatis growth and disturb chromosome replication. Front Microbiol. 2018;9:1592–605. https://doi.org/10.3389/fmicb.2018.01592
Beck DE, Reddy PV, Lv W, Abdelmalak M, Tender GS, Lopez S, et al. Investigation of the structure-activity relationships of aza-A-ring indenoisoquinoline topoisomerase I poisons. J Med Chem. 2016;59:3840–53. https://doi.org/10.1021/acs.jmedchem.6b00003
Elsayed MSA, Su Y, Wang P, Sethi T, Agama K, Ravji A, et al. Design and synthesis of chlorinated and fluorinated 7-azaindenoisoquinolines as potent cytotoxic anticancer agents that inhibit topoisomerase I. J Med Chem. 2017;60:5364–76. https://doi.org/10.1021/acs.jmedchem.6b01870
Wang S, Fang K, Dong G, Chen S, Liu N, Miao Z, et al. Scaffold diversity inspired by the natural product evodiamine: discovery of highly potent and multitargeting antitumor agents. J Med Chem. 2015;58:6678–96. https://doi.org/10.1021/acs.jmedchem.5b00910
Ma ZZ, Hano Y, Nomura T, Chen YJ. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles. 1997;46:541–6. https://doi.org/10.3987/COM-97-S65
Cagir A, Jones SH, Gao R, Eisenhauer BM, Hecht SM. Luotonin A. A naturally occurring human DNA topoisomerase I poison. J Am Chem Soc. 2003;125:13628–9. https://doi.org/10.1021/ja0368857
Almansour AI, Arumugam N, Suresh Kumar R, Mahalingam SM, Sau S, Bianchini G, et al. Design, synthesis and antiproliferative activity of decarbonyl luotonin analogues. Eur J Med Chem. 2017;138:932–41. https://doi.org/10.1016/j.ejmech.2017.07.027
Domagala JM, Hanna LD, Heifetz CL, Hutt MP, Mich TF, Sanchez JP, et al. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986;29:394–404. https://doi.org/10.1021/jm00153a015
Delgado JL, Lentz SRC, Kulkarni CA, Chheda PR, Held HA, Hiasa H, et al. Probing structural requirements for human topoisomerase I inhibition by a novel N1-biphenyl fluoroquinolone. Eur J Med Chem. 2019;172:109–30. https://doi.org/10.1016/j.ejmech.2019.03.040
Oppegard LM, Delgado JL, Kulkarni CA, Towle TR, Hart DE, Williams BP, et al. Novel N-1 substituted fluoroquinolones inhibit human topoisomerase I activity and exhibit anti-proliferative activity. Invest New Drugs. 2019;37:378–83. https://doi.org/10.1007/s10637-018-0666-x
Ge R, Zhao Q, Xie Z, Lu L, Guo Q, Li Z, et al. Synthesis and biological evaluation of 6-fluoro-3-phenyl-7-piperazinyl quinolone derivatives as potential topoisomerase I inhibitors. Eur J Med Chem. 2016;122:465–74. https://doi.org/10.1016/j.ejmech.2016.06.054
Ye R, Cao Y, Xi X, Liu L, Chen T. Metal- and radical-free aerobic oxidation of heteroaromatic methanes: an efficient synthesis of heteroaromatic aldehydes. Org Biomol Chem. 2019;17:4220–4. https://doi.org/10.1039/c9ob00490d
Yang L, Shi X, Hu BQ, Wang LX. Iodine-catalyzed oxidative benzylic C-H bond amination of azaarenes: practical synthesis of quinazolin-4(3H)-ones. Asian J Org Chem. 2016;5:494–8. https://doi.org/10.1002/ajoc.201600041
Kwon SH, Seo HA, Cheon CH. Total synthesis of luotonin A and rutaecarpine from an aldimine via the designed cyclization. Org Lett. 2016;18:5280–3. https://doi.org/10.1021/acs.orglett.6b02597
Wang X, Zhou CX, Yan JW, Hou JQ, Chen SB, Ou TM, et al. Synthesis and evaluation of quinazolone derivatives as a new class of c-KIT G-quadruplex binding ligands. ACS Med Chem Lett. 2013;4:909–14. https://doi.org/10.1021/ml400271y
Wang YQ, Huang ZL, Chen SB, Wang CX, Shan C, Yin QK, et al. Design, synthesis, and evaluation of new selective NM23-H2 binders as c-MYC transcription inhibitors via disruption of the NM23-H2/G-quadruplex interaction. J Med Chem. 2017;60:6924–41. https://doi.org/10.1021/acs.jmedchem.7b00421
Che T, Chen SB, Tu JL, Wang B, Wang YQ, Zhang Y, et al. Discovery of novel schizocommunin derivatives as telomeric G-quadruplex ligands that trigger telomere dysfunction and the deoxyribonucleic acid (DNA) damage response. J Med Chem. 2018;61:3436–53. https://doi.org/10.1021/acs.jmedchem.7b01615
Shemchuk LA, Chernykh VP, Krys’kiv OS. Reaction of anthranilic acid amides with cyclic anhydrides. Russ J Org Chem. 2006;42:382–7. https://doi.org/10.1134/s1070428006030079
Nathubhai A, Haikarainen T, Hayward PC, Muñoz-Descalzo S, Thompson AS, Lloyd MD, et al. Structure-activity relationships of 2-arylquinazolin-4-ones as highly selective and potent inhibitors of the tankyrases. Eur J Med Chem. 2016;118:316–27. https://doi.org/10.1016/j.ejmech.2016.04.041
Liu G, Chao Q, Hadd MJ, Holladay MW, Abraham S, Setti E. Jak kinase modulating quinazoline derivatives and methods of use thereof. WO2010099379. 2010.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. https://doi.org/10.1002/jcc.21256
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. https://doi.org/10.1186/1758-2946-3-33
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009;31:455–61. https://doi.org/10.1002/jcc.21334
Acknowledgements
This work was supported by the Scientific Research Project of Education Department of Hubei Province (D20192003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiang, Y., Li, H., Wang, J. et al. Design, synthesis, and anticancer activities of 8,9-substituted Luotonin A analogs as novel topoisomerase I inhibitors. Med Chem Res 30, 1512–1522 (2021). https://doi.org/10.1007/s00044-021-02749-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02749-y