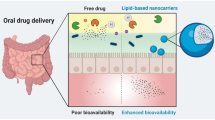
Abstract
Purpose
Successful oral peptide delivery faces two major hurdles: low enzymatic stability in the gastro-intestinal lumen and poor intestinal membrane permeability. While lipid-based formulations (LBF) have the potential to overcome these barriers, effective formulation of peptides remains challenging. Lipophilic salt (LS) technology can increase the apparent lipophilicity of peptides, making them more suitable for LBF.
Methods
As a model therapeutic peptide, octreotide (OCT) was converted to the docusate LS (OCT.DoS2), and compared to the commercial acetate salt (OCT.OAc2) in oral absorption studies and related in vitro studies, including parallel artificial membrane permeability assay (PAMPA), Caco-2, in situ intestine perfusion, and simulated digestion in vitro models. The in vivo oral absorption of OCT.DoS2 and OCT.OAc2 formulated in self-emulsifying drug delivery systems (SEDDS) was studied in rats.
Results
LS formulation improved the solubility and loading of OCT in LBF excipients and OCT.DoS2 in combination with SEDDS showed higher OCT absorption than the acetate comparator in the in vivo studies in rats. The Caco-2 and in situ intestine perfusion models indicated no increases in permeability for OCT.DoS2. However, the in vitro digestion studies showed reduced enzymatic degradation of OCT.DoS2 when formulated in the SEDDS formulations. Further in vitro dissociation and release studies suggest that the enhanced bioavailability of OCT from SEDDS-incorporating OCT.DoS2 is likely a result of higher partitioning into and prolonged retention within lipid colloid structures.
Conclusion
The combination of LS and LBF enhanced the in vivo oral absorption of OCT primarily via the protective effect of LBF sheltering the peptide from gastrointestinal degradation.








Similar content being viewed by others
References
Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–35.
Bak A, Leung D, Barrett SE, Forster S, Minnihan EC, Leithead AW, et al. Physicochemical and formulation developability assessment for therapeutic peptide delivery--a primer. AAPS J. 2015;17(1):144–55.
Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2020;5(2):127–48.
Zhang Y, Zhang H, Ghosh D, Williams RO 3rd. Just how prevalent are peptide therapeutic products? A critical review. Int J Pharm. 2020;587:119491.
Duran-Lobato M, Niu Z, Alonso MJ. Oral delivery of biologics for precision medicine. Adv Mater. 2020;32(13):e1901935.
Drucker DJ. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19(4):277–89.
Brayden DJ, Hill TA, Fairlie DP, Maher S, Mrsny RJ. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv Drug Deliv Rev. 2020;157:2–36.
Anderson SL, Beutel TR, Trujillo JM. Oral semaglutide in type 2 diabetes. J Diabetes Complicat. 2020;34(4):107520.
Melmed S, Popovic V, Bidlingmaier M, Mercado M, van der Lely AJ, Biermasz N, et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J Clin Endocrinol Metab. 2015;100(4):1699–708.
Tyagi P, Pechenov S, Anand SJ. Oral peptide delivery: translational challenges due to physiological effects. J Control Release. 2018;287:167–76.
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.
Boyd BJ, Bergstrom CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;137:104967.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Gupta S, Jain A, Chakraborty M, Sahni JK, Ali J, Dang S. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Deliv. 2013;20(6):237–46.
Williams HD, Ford L, Igonin A, Shan Z, Botti P, Morgen MM, et al. Unlocking the full potential of lipid-based formulations using lipophilic salt/ionic liquid forms. Adv Drug Deliv Rev. 2019;142:75–90.
Nazir I, Asim MH, Dizdarevic A, Bernkop-Schnurch A. Self-emulsifying drug delivery systems: impact of stability of hydrophobic ion pairs on drug release. Int J Pharm. 2019;561:197–205.
Bonengel S, Jelkmann M, Abdulkarim M, Gumbleton M, Reinstadler V, Oberacher H, et al. Impact of different hydrophobic ion pairs of octreotide on its oral bioavailability in pigs. J Control Release. 2018;273:21–9.
Choi SH, Park TG. Hydrophobic ion pair formation between leuprolide and sodium oleate for sustained release from biodegradable polymeric microspheres. Int J Pharm. 2000;203(1–2):193–202.
Shahzadi I, Nazir I, Nhu Quynh Phan T, Bernkop-Schnurch A. About the impact of superassociation of hydrophobic ion pairs on membrane permeability. Eur J Pharm Biopharm. 2020.
Hintzen F, Perera G, Hauptstein S, Muller C, Laffleur F, Bernkop-Schnurch A. In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin. Int J Pharm. 2014;472(1–2):20–6.
Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem. 2001;44(6):923–30.
Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJ. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30(12):2976–92.
Yeap YY, Trevaskis NL, Quach T, Tso P, Charman WN, Porter CJ. Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol Pharm. 2013;10(5):1874–89.
Sahbaz Y, Nguyen TH, Ford L, McEvoy CL, Williams HD, Scammells PJ, et al. Ionic liquid forms of weakly acidic drugs in oral lipid formulations: preparation, characterization, in vitro digestion, and in vivo absorption studies. Mol Pharm. 2017;14(11):3669–83.
Sahbaz Y, Williams HD, Nguyen TH, Saunders J, Ford L, Charman SA, et al. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm. 2015;12(6):1980–91.
Bilton M, Brown AP, Milne SJ. Investigating the optimum conditions for the formation of calcium oxide, used for CO2 sequestration, by thermal decomposition of calcium acetate. J Phys Conf Ser. 2012;371:012075.
Crum MF, Trevaskis NL, Williams HD, Pouton CW, Porter CJ. A new in vitro lipid digestion - in vivo absorption model to evaluate the mechanisms of drug absorption from lipid-based formulations. Pharm Res. 2016;33(4):970–82.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Samiei N, Shafaati A, Zarghi A, Moghimi HR, Foroutan SM. Enhancement and in vitro evaluation of amifostine permeation through artificial membrane (PAMPA) via ion pairing approach and mechanistic selection of its optimal counter ion. Eur J Pharm Sci. 2014;51:218–23.
Miller JM, Dahan A, Gupta D, Varghese S, Amidon GL. Enabling the intestinal absorption of highly polar antiviral agents: ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir. Mol Pharm. 2010;7(4):1223–34.
Marrucho I, Branco L, Rebelo L. Ionic liquids in pharmaceutical applications. Ann Rev Chem Biomol Eng. 2014;5:527–46.
Agatemor C, Ibsen KN, Tanner EE, Mitragotri S. Ionic liquids for addressing unmet needs in healthcare. Bioeng Transl Med. 2018;3(1):7–25.
Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117(10):7132–89.
Ristroph KD, Prud'homme RK. Hydrophobic ion pairing: encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019;1(11):4207–37.
Fuessl HS, Domin J, Bloom SR. Oral absorption of the somatostatin analogue SMS 201-995: theoretical and practical implications. Clin Sci (Lond). 1987;72(2):255–7.
Salman K, Rose LI. Octreotide: a somatostatin analog. Am Fam Physician. 1989;39(6):207–11.
Dorkoosh FA, Broekhuizen CA, Borchard G, Rafiee-Tehrani M, Verhoef JC, Junginger HE. Transport of octreotide and evaluation of mechanism of opening the paracellular tight junctions using superporous hydrogel polymers in Caco-2 cell monolayers. J Pharm Sci. 2004;93(3):743–52.
Thanou M, Verhoef JC, Marbach P, Junginger HE. Intestinal absorption of octreotide: N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivo. J Pharm Sci. 2000;89(7):951–7.
Fricker G, Drewe J, Vonderscher J, Kissel T, Beglinger C. Enteral absorption of octreotide. Br J Pharmacol. 1992;105(4):783–6.
Fedorak RN, Allen SL. Effect of somatostatin analog (SMS 201-995) on in vivo intestinal fluid transport in rats. A limited systemic effect. Dig Dis Sci. 1989;34(4):567–72.
Thotakura N, Kaushik L, Kumar V, Preet S, Babu PV. Advanced approaches of bioactive peptide molecules and protein drug delivery systems. Curr Pharm Des. 2018;24(43):5147–63.
Hetenyi G, Griesser J, Moser M, Demarne F, Jannin V, Bernkop-Schnurch A. Comparison of the protective effect of self-emulsifying peptide drug delivery systems towards intestinal proteases and glutathione. Int J Pharm. 2017;523(1):357–65.
Zupancic O, Leonaviciute G, Lam HT, Partenhauser A, Podricnik S, Bernkop-Schnurch A. Development and in vitro evaluation of an oral SEDDS for desmopressin. Drug Deliv. 2016;23(6):2074–83.
Leonaviciute G, Zupancic O, Prufert F, Rohrer J, Bernkop-Schnurch A. Impact of lipases on the protective effect of SEDDS for incorporated peptide drugs towards intestinal peptidases. Int J Pharm. 2016;508(1–2):102–8.
Devraj R, Williams HD, Warren DB, Porter CJ, Pouton CW. Choice of nonionic surfactant used to formulate type IIIA self-emulsifying drug delivery systems and the physicochemical properties of the drug have a pronounced influence on the degree of drug supersaturation that develops during in vitro digestion. J Pharm Sci. 2014;103(4):1050–63.
ACKNOWLEDGMENTS AND DISCLOSURES
We kindly thank Dr. Jason Dang for acquiring all HRMS data and Dr. Kasiram Katneni for support with Caco-2 studies. This research article describes intellectual property in the use of ionic liquids/lipophilic salts in drug delivery that has been assigned to Lonza Group. Authors Peng Li, Leigh Ford, Hywel D. Williams, Vincent Jannin, Hassan Benameur were from Lonza Group.
Funding
Funding support was provided by Lonza Group.
Author information
Authors and Affiliations
Contributions
Peng Li, Hywel D. Williams, Peter J. Scammells, Philip Thompson, Vincent Jannin, Christopher J. H. Porter, Hassan Benameur, Colin Pouton substantially contributed to the conception and design of the work.
Peng Li, Leigh Ford, Shadabul Haque, Mitchell P. McInerney, Hywel D. Williams, Peter J. Scammells, Philip Thompson, Vincent Jannin, Christopher J. H. Porter, Hassan Benameur, Colin Pouton, substantially contributed to the acquisition, analysis, and interpretation of data for the work.
Peng Li, Leigh Ford, Shadabul Haque, Mitchell P. McInerney, Hywel D. Williams, Vincent Jannin, Christopher J. H. Porter, substantially contributed to the draft manuscript and critically revised the manuscript.
All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 173 kb)
Rights and permissions
About this article
Cite this article
Li, P., Ford, L., Haque, S. et al. Lipophilic Salts and Lipid-Based Formulations: Enhancing the Oral Delivery of Octreotide. Pharm Res 38, 1125–1137 (2021). https://doi.org/10.1007/s11095-021-03063-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03063-3