Abstract
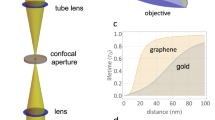
Super-resolution fluorescence imaging that surpasses the classical optical resolution limit is widely utilized for resolving the spatial organization of biological structures at molecular length scales. In one example, single-molecule localization microscopy, the lateral positions of single molecules can be determined more precisely than the diffraction limit if the camera collects individual photons separately. Using several schemes that introduce engineered optical aberrations in the imaging optics, super-resolution along the optical axis (perpendicular to the sample plane) has been achieved, and single-molecule localization microscopy has been successfully applied for the study of 3D biological structures. Nonetheless, the achievable axial localization accuracy is typically three to five times worse than the lateral localization accuracy. Only a few exceptional methods based on interferometry exist that reach nanometer 3D super-resolution, but they involve enormous technical complexity and restricted sample preparations that inhibit their widespread application. We developed metal-induced energy transfer imaging for localizing fluorophores along the axial direction with nanometer accuracy, using only a conventional fluorescence lifetime imaging microscope. In metal-induced energy transfer, experimentally measured fluorescence lifetime values increase linearly with axial distance in the range of 0–100 nm, making it possible to calculate their axial position using a theoretical model. If graphene is used instead of the metal (graphene-induced energy transfer), the same range of lifetime values occurs over a shorter axial distance (~25 nm), meaning that it is possible to get very accurate axial information at the scale of a membrane bilayer or a molecular complex in a membrane. Here, we provide a step-by-step protocol for metal- and graphene-induced energy transfer imaging in single molecules, supported lipid bilayer and live-cell membranes. Depending on the sample preparation time, the complete duration of the protocol is 1–3 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data files corresponding to Figs. 3–6 can be downloaded from https://data.goettingen-research-online.de/dataset.xhtml?persistentId=doi:10.25625/NIDERQ.
Code availability
A custom-written open-source MATLAB-based MIET GUI is available from https://projects.gwdg.de/projects/miet/repository/raw/MIET_GUI.zip?rev=ZIP. The MATLAB-based software package for fluorescence lifetime fitting is available free of charge at https://www.joerg-enderlein.de/software.
References
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Hess, S. T., Girirajan, T. P. & Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 47, 6172–6176 (2008).
Sharonov, A. & Hochstrasser, R. M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl Acad. Sci. USA 103, 18911–18916 (2006).
Schnitzbauer, Joerg et al. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 12.6, 1198 (2017).
Balzarotti, F. et al. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 355, 606–612 (2016).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Gwosch, K. C. et al. MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells. Nat. Methods 17, 217–224 (2020).
Shtengel, G. et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl Acad. Sci. USA 106, 3125–3130 (2009).
Aquino, D. et al. Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores. Nat. Methods 8, 353–359 (2011).
Schmidt, R. et al. Spherical nanosized focal spot unravels the interior of cells. Nat. Methods 5, 539–544 (2008).
Hell, S. W., Schmidt, R. & Egner, A. Diffraction-unlimited three-dimensional optical nanoscopy with opposing lenses. Nat. Photonics 3, 381–387 (2009).
Juette, M. F. et al. Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods 5, 527–529 (2008).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional superresolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Backlund, M. P. et al. Simultaneous, accurate measurement of the 3D position and orientation of single molecules. Proc. Natl Acad. Sci. USA 109, 19087–19092 (2012).
Santos, D. et al. Topography of cells revealed by variable-angle total internal reflection fluorescence microscopy. Biophys. J. 111, 1316–1327 (2016).
Loerke, D., Stühmer, W. & Oheim, M. Quantifying axial secretory-granule motion with variable-angle evanescent-field excitation. J. Neurosci. Methods 119, 65–73 (2002).
Winterflood, C. M. et al. Nanometer axial resolution by three-dimensional supercritical angle fluorescence microscopy. Phys. Rev. Lett. 105, 108103 (2010).
Deschamps, J., Mund, M. & Ries, J. 3D superresolution microscopy by supercritical angle detection. Opt. Express, 22, 29081–29091 (2014).
Chizhik, A. I., Rother, J., Gregor, I., Janshoff, A. & Enderlein, J. Metal-induced energy transfer for live cell nanoscopy. Nat. Photonics 8, 124–127 (2014).
Karedla, N. et al. Single-molecule metal-induced energy transfer (smMIET): resolving nanometer distances at the single-molecule level. ChemPhysChem 15, 705–711 (2014).
Ghosh, A. et al. Graphene-based metal-induced energy transfer for sub-nanometre optical localization. Nat. Photonics 13, 860–865 (2019).
Roy, R., Hohng, S. & Ha, T. A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 (2008).
Baronsky, T. et al. Cell–substrate dynamics of the epithelial-to-mesenchymal transition. Nano Lett. 17, 3320–3326 (2017).
Chance, R., Prock, A. & Silbey, R. Molecular fluorescence and energy transfer near interfaces. Adv. Chem. Phys. 37, 1–65 (1978).
Enderlein, J. Single-molecule fluorescence near a metal layer. Chem. Phys. 247, 1–9 (1999).
Gregor, I. et al. Metal-induced energy transfer. Nanophotonics 8, 1689–1699 (2019).
Chizhik, A. I. & Enderlein, J. Metal-induced energy transfer imaging. in Nanoscale Photonic Imaging (eds Salditt, T. et al.) 227–239 (Springer, 2020).
Simoncelli, S., Makarova, M., Wardley, W. & Owen, D. M. Toward an axial nanoscale ruler for fluorescence microscopy. ACS Nano 11, 11762–11767 (2017).
Chizhik, A. M. et al. Dual-color metal-induced and Förster resonance energy transfer for cell nanoscopy. Mol. Biol. Cell 29, 846–851 (2018).
Chizhik, A. M. et al. Three-dimensional reconstruction of nuclear envelope architecture using dual-color metal-induced energy transfer imaging. ACS Nano 11, 11839–11846 (2017).
Zelená, A. et al. Time-resolved MIET measurements of blood platelet spreading and adhesion. Nanoscale 12, 21306–21315 (2020).
Isbaner, S. et al. Axial colocalization of single molecules with nanometer accuracy using metal-induced energy transfer. Nano Lett. 18, 2616–2622 (2018).
Karedla, N. et al. Three-dimensional single-molecule localization with nanometer accuracy using metal-induced energy transfer (MIET) imaging. J. Chem. Phys. 148, 204201 (2018).
Moerland, R. J. & Hoogenboom, J. P. Subnanometer-accuracy optical distance ruler based on fluorescence quenching by transparent conductors. Optica 3, 112–117 (2016).
Wei, D. et al. Ultrathin rechargeable all-solid-state batteries based on monolayer graphene. J. Mater. Chem. A 1, 3177–3181 (2013).
Chizhik, A. I., Gregor, I., Ernst, B. & Enderlein, J. Nanocavity-based determination of absolute values of photoluminescence quantum yields. ChemPhysChem 14, 505–513 (2013).
Schneider, F., Ruhlandt, D., Gregor, I., Enderlein, J. & Chizhik, A. I. Quantum yield measurements of fluorophores in lipid bilayers using a plasmonic nanocavity. J. Phys. Chem. Lett. 8, 1472–1475 (2017).
Oleksiievets, N. et al. Wide-field fluorescence lifetime imaging of single molecules. J. Phys. Chem. A 124, 3494–3500 (2020).
Maruyama, Y. & Charbon, E. An all-digital, time-gated 128X128 spad array for on-chip, filter-less fluorescence detection. 16th International Solid-State Sensors, Actuators and Microsystems Conference, 1180-1183. (2011)
Böhmer, M. & Enderlein, J. Orientation imaging of single molecules by wide-field epifluorescence microscopy. J. Opt. Soc. Am. B 20, 554–559 (2003).
Patra, D., Gregor, I. & Enderlein, J. Image analysis of defocused single molecule images for three-dimensional molecule orientation studies. J. Phys. Chem. A 108, 6836 (2004).
Backer, A. S. & Moerner, W. E. Determining the rotational mobility of a single molecule from a single image: a numerical study. Opt. Express 23, 4255–4276 (2015).
Isbaner, S. et al. Dead-time correction of fluorescence lifetime measurements and fluorescence lifetime imaging. Opt. Express 24, 9429–9445 (2016).
GWDG Project Management Service. https://projects.gwdg.de/projects/miet/repository/raw/MIET_GUI.zip?rev=ZIP
Richter, R. P., Bérat, R. & Brisson, A. R. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 22, 3497–3505 (2006).
Meleard, P., Bagatolli, L. A. & Pott, T. Giant unilamellar vesicle electroformation: from lipid mixtures to native membranes under physiological conditions. Methods Enzymol. 465, 161–176 (2009).
Acknowledgements
N.K. and A.G. are grateful to the Deutsche Forschungsgemeinschaft (DFG) for funding via project A06 of the Collaborative Research Centre SFB 860. J.E. acknowledges funding by the DFG under Germany’s Excellence Strategy—EXC 2067/1- 390729940, and financing by the European Research Council (ERC) via project ‘smMIET’ (grant agreement 884488) under the European Union’s Horizon 2020 research and innovation program.
Author information
Authors and Affiliations
Contributions
J.E. conceived the project and helped with data analysis; A.G., A.I.C. and N.K. performed experiments and analyzed the data; A.G. and J.E. wrote the manuscript with the input of all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Yuval Garini and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Chizhik, A. I. et al. Nat. Photonics 8, 124–127 (2014): https://doi.org/10.1038/nphoton.2013.345
Karedla, N. et al. ChemPhysChem 15, 705–711 (2014): https://doi.org/10.1002/cphc.201300760
Ghosh, A. et al. Nat. Photonics 13, 860–865 (2019): https://doi.org/10.1038/s41566-019-0510-7
Supplementary information
Supplementary Information
Supplementary Notes 1–4 and Supplementary Fig. 1.
Rights and permissions
About this article
Cite this article
Ghosh, A., Chizhik, A.I., Karedla, N. et al. Graphene- and metal-induced energy transfer for single-molecule imaging and live-cell nanoscopy with (sub)-nanometer axial resolution. Nat Protoc 16, 3695–3715 (2021). https://doi.org/10.1038/s41596-021-00558-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00558-6
This article is cited by
-
An alternative to MINFLUX that enables nanometer resolution in a confocal microscope
Light: Science & Applications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.