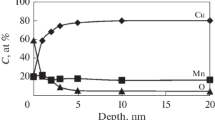
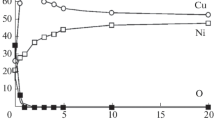
Abstract
The chemical composition and structure of the Cu–Mn alloy after modification with oxygen ions with an energy of 10–30 keV and a dose of 1017 cm–2 have been investigated. Changes in the type of chemical bonding and atomic structure in irradiated Cu–Mn samples have been studied by X-ray photoelectron spectroscopy and X-ray diffraction. Electrochemical studies of ion-modified surfaces have been performed by measuring the anodic potentiodynamic polarization curves in a neutral borate buffer solution, hydrochloric acid, and potassium hydroxide.








Similar content being viewed by others
REFERENCES
Skorchelletti, V.V., Theory of Metal Corrosion, IPST, 1977.
Tomashov, N.D. and Chernova, G.P., Teoriya korrozii i korrozionnostoikie konstruktsionnye splavy (Theory of Corrosion and Corrosion-Resistant Structural Alloys), Moscow: Metallurgiya, 1993.
Semenova, I.V., Florianovich, G.M., and Khoroshilov, A.V., Korroziya i zashchita ot korrozii (Corrosion and Corrosion Protection), Moscow: Fizmatlit, 2002.
Kolotyrkin, V.I. and Knyazheva, V.M., Possibilities of high-energy metal surface treatment methods for corrosion protection, Zashch. Met., 1991, vol. 27, no. 2, pp. 184–186.
Vasil’ev, V.Yu., Betuganov, M.A., Isaev, N.I., Kuzmenko, T.G., Yakovlev, V.B., and Shumilov, V.N., Effect of ion implantation on the electrochemical characteristics of alloys, Zashch. Met., 1981, vol. 12, no. 5, pp. 543–545.
Parshutin, V.V. and Pyshkin, S.L., Investigation of the properties of modified steel surface, Zashch. Met., 1994, vol. 30, no. 3, pp. 276–281.
Reshetnikov, S.M., Gil’mutdinov, F.Z., Borisova, E.M., Bakieva, O.R., and Vorob’ev, V.L., Effect of oxygen implantation of corrosion-electrochemical properties of copper, Korroz.: Mater. Zashchit., 2017, no. 9, pp. 21–30.
Corrosion, Shreir, L.L., Ed., London–Boston: Butterworth-Heinemann, 1976.
Todt, F., Korrosion und Korrosionsschutz, Berlin: Walter de Gruyter, 1961.
Marshakov, I.K., Vvedenskii, A.V., Kondrashin, V.Yu., and Bokov, G.A., Anodnoe rastvorenie i selektivnaya korroziya splavov (Anodic Dissolution and Selective Corrosion of Alloys), Voronezh: Voronezh State Univ., 1988.
Dvoinye i mnogokomponentnye sistemy na osnove medi (Copper-Based Binary and Multicomponent Systems), Shukhardin, S.V., Ed., Moscow: Nauka, 1979.
Akhmadullina, A.G., Abdrakhimov, Yu.R., and Smirnov, I.N., Obezvrezhivanie i ispol’zovanie sernisto-shchelochnykh otkhodov neftepererabotki i neftekhimii (Neutralization and Use of Sulfur-Alkaline Waste Oil Refining and Petrochemistry), Moscow: TsNIITEnefte-khim, 1990, no. 4.
Hoffinan, M.R. and Lim, B.C., Kinetics and mechanism of the oxidation of sulfide by oxygen: Catalysis by homogeneous metal-phthalocyanine complexes, Environ. Sci. Technol., 1979, vol. 13, no. 11, pp. 1406–1414.
Akhmadullin, R.M., Bui, D.N., and Akhmadullina, A.G., The study of binary mixed compositions of transition metal oxides deposited on the polymer matrix in the sodium sulfide oxidation, Butlerov. Soobshch., 2012, vol. 30, no. 5, pp. 94–97.
Geol. Survey Jpn. Open File Report no. 419: Atlas of Eh–pH Diagrams. Intercomparison of Thermodynamic Databases, Naoto, Takeno: Nat. Inst. Adv. Indust. Sci. Technol., Res. Center for Deep Geol. Environ., 2005, pp. 85–87. http://www.eosremediation.com/download/Chemistry/Chemical%20Properties/Eh_pH_ Diagrams.pdf.
Surnin, D.V., Vorobiev, V.L., Gilmutdinov, F.Z., et al., Chemical composition and atomic structure of the surface of copper-manganese alloy modified with oxygen ions, J. Surf. Invest.: X-Ray, Synchrotron Neutron Tech., 2016, vol. 10, no. 2, pp. 433–437.
Nefedov, V.I., Rentgenoelektronnaya spektroskopiya khimicheskikh soedinenii (X-Ray Photoelectron Spectroscopy of Chemical Compounds), Moscow: Khimiya, 1984.
Briggs, D. and Seah, M., Auger and X-ray Photoelectron Spectroscopy, Chichester: Wiley, 1983.
Naumov, G.B., Ryzhenko, B.N., and Khodakovsky, I.L., Handbook of Thermodynamic Data, Menlo Park, California: U.S. Geol. Survey, Water Res. Div., 1974.
ACKNOWLEDGMENTS
This work was performed using the equipment of the Shared Center for Physical and Physicochemical Methods of Analysis, Study of Properties and Characteristics of Surface, Nanostructures, Materials, and Products of the Udmurt Federal Research Center, Ural Branch, Russian Academy of Sciences.
Funding
This work was performed under the research grant no. АААА-А17-117022250040-0 with the partial financial support of the Russian Foundation for Basic Research, project no. 14-02-31488, and the Presidium of the Russian Academy of Sciences, project no. 18-10-2-25.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Kharitonov
Rights and permissions
About this article
Cite this article
Vorobiev, V.L., Bakieva, O.R., Gilmutdinov, F.Z. et al. Effect of Oxygen Ion Implantation on Physicochemical Structure and Corrosion-Electrochemical Behavior of Copper–Manganese Alloy. Inorg. Mater. Appl. Res. 12, 581–587 (2021). https://doi.org/10.1134/S2075113321030394
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321030394