Abstract
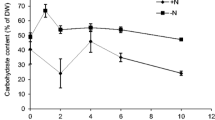
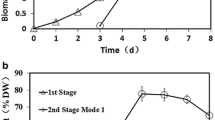
Microalgae are considered promising feedstocks for biofuel and bio-product generation. The algal carbohydrates can be hydrolyzed into sugars before their fermentation into ethanol. In this study, nutrient limitation strategy was employed to evaluate the biochemical composition of Chlorella sorokiniana. Limiting nitrate (1.0 g/L KNO3) in the culture medium increased the total carbohydrate and starch content of microalga by 50.28 and 34.06%, respectively. However, this significantly lowered their yield due to low microalgal biomass production. Cultivation of C. sorokiniana cells with 4.0 g/L KNO3 as nitrogen source for 8 days was optimum for bioethanol production as the highest total carbohydrate yield of 422.44 mg/L was obtained under these conditions. Nitrate limitation (1.0 g/L KNO3) favored the increased production of high-value carotenoids in C. sorokiniana that could further contribute to improving the economics of the bioethanol production process. Feasibility studies for ethanol production from C. sorokiniana revealed that a maximum of 13.86 mg/mL of reducing sugars was extracted in the hydrolysate by treating the microalgal biomass with 2.8% sulfuric acid at 121 °C for 30 min. Fermentation of acid hydrolysate produced ethanol at a concentration of 2.91 mg/mL in 96 h with 41.16% of theoretical yield.





Similar content being viewed by others
References
Velazquez-Lucio J, Rodríguez-Jasso RM, Colla LM, Saenz-Galindo A, Cervantes-Cisneros DE, Aguilar CN, Fernandes BD, Ruiz HA (2018) Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J 5:780–791. https://doi.org/10.18331/BRJ2018.5.1.5
Chen CY, Zhao XQ, Yen HW, Ho SH, Chang CL, Lee DJ, Bai FW, Chang JS (2013) Microalgae- based carbohydrates for biofuel production. Biochem Eng J 78:1–10. https://doi.org/10.1016/j.bej.2013.03.006
Garcia LM, Adjalle K, Barnabe S, Ragha GSV (2017) Microalgae biomass production for a biorefinery system: recent advances and the way towards sustainability. Renew Sust Energ Rev 76:493–506. https://doi.org/10.1016/j.rser.2017.03.024
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6(9):4607–4638
Agwa OK, Nwosu IG, Abu GO (2017) Bioethanol production from Chlorella vulgaris biomass cultivated with Plantain (Musa paradisiaca) pells abstract. Adv Biosci Biotechnol 8:478–490. https://doi.org/10.4236/abb.2017.812035
Barkia I, Saar N, Manning SR (2019) Microalgae for high-value products towards human health and nutrition. Mar Drugs 17(5):1–29
Branyikova I, Marsalkova B, Doucha J, Branyik T, Bisova K, Zachleder V (2011) Microalgae - novel highly efficient starch producers. Biotechnol Bioeng 108:766–776. https://doi.org/10.1002/bit.23016
Zhu S, Wang Y, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Enhanced accumulation of carbohydrate and starch in Chlorella zofingiensis induced by starvation. Appl Biochem Biotechnol 174:2435–2445. https://doi.org/10.1007/s12010-014-1183-9
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Behrens PW, Bingham SE, Hoeksema SD, Cohoon DL, Cox JC (1989) Studies on the incorporation of CO2 into starch by Chlorella vulgaris. J Appl Phycol 1:123–130. https://doi.org/10.1007/BF00003874
Ueda R, Hirayama S, Sugata K, Nakayama H (1996) Process for the production of ethanol from microalgae. US Patent 5,578,472
Barros AI, Goncalves AL, Simoes M, Pires JCM (2015) Harvesting techniques applied to microalgae: a review. Renew Sust Energ Rev 41:1489–1500. https://doi.org/10.1016/j.rser.2014.09.037
Phwan CK, Chew KW, Sebayang AH, Ong HC, Ling TC, Malek MA, Show PL (2019) Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol Biofuels 12:191. https://doi.org/10.1186/s13068-019-1533-5
Harun R, Danquah MK (2011) Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem 46(1):304–309. https://doi.org/10.1016/j.procbio.2010.08.027
Bojórquez NV, Rocha RV, Escalante MAA, Barajas JAS (2016) Production of bioethanol from biomass of microalgae Dunaliella tertiolecta. Int J Env Agri Res 2(2):110–116
Agustini NWS, Hidhayati N, Wibisono SA (2019) Effect of hydrolysis time and acid concentration on bioethanol production of microalgae Scenedesmus sp. IOP Conf Series Earth Environ Sci 308:1–11
Duarte LC, Fernandes TS, Carvalheiro F, Girio FM (2009) Dilute acid hydrolysis of wheat straw oligosaccharides. Appl Biochem Biotechnol 153(1):116–126
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78(1):29–36. https://doi.org/10.1007/s00253-007-1285-1
Yao CH, Ai JN, Cao XP, Xue S (2013) Salinity manipulation as an effective method for enhanced starch production in the marine microalga Tetraselmis subcordiformis. Bioresour Technol 146(6):663–671. https://doi.org/10.1016/j.biortech.2013.07.134
Margarites ACF, Costa JAV (2014) Increment of carbohydrate concentration of Chlorella minutissina microalgae for bioethanol production. Int J Res Appl 4(3):80–86
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Kong WB, Yang H, Cao YT, Song H, Hua SF, Xia CG (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotropic culture. Food Technol Biotechnol 51(1):62–69
Lowry OH, Rosenbrough NL, Farr AL, Radall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193(1):265–275
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protocols Food Anal Chem 1:F4.3.1-F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Nelson N (1994) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Markou G, Angelidaki I, Nerantis E, Georgakakis D (2013) Bioethanol production by carbohydrate-enriched biomass Arthrospira (Spirulina) platensis. Energy 6:3937–3950. https://doi.org/10.3390/en6083937
Caputi AJ, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Viticult 19(3):160–165
Suyono EA, Muavatun U, Husna F, Khotimah H, Pratiwi I, Husna R, Cahyani F, Purwanti Y, Samudra TT (2016) The effect of nitrogen stress in medium for increasing carbohydrate as a bioethanol source and carotenoid as an antioxidant from Chlorella zofingiensis culture. ARPN J Eng Appl Sci 11:2698–2701
Negi S, Barry AN, Friedland N, Sudasinghe N, Subramanian S, Pieris S, Holguin FO, Dungan B, Schaub T, Sayre R (2015) Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J Appl Phycol 28:803–812. https://doi.org/10.1007/s10811-015-0652-z
Mollers KB, Cannella D, Jorgensen H, Frigaard NU (2014) Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol Biofuels 7(1):64. https://doi.org/10.1186/1754-6834-7-64
Recht L, Zarka A, Boussiba S (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechnol 94:1495–1503. https://doi.org/10.1007/s00253-012-3940-4
Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Ann Rev Plant Biol 54:207–233
Michelon W, Da Silva ML, Mezzari MP, Pirolli M, Prandini JM, Soares HM (2016) Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggery wastewater digestate. Applied Biochem Biotechnol 178(7):1407–1419. https://doi.org/10.1007/s12010-015-1955-x
Vello V, Chu WL, Lim PE, Majid NA, Phang SM (2018) Metabolomic profiles of tropical Chlorella species in response to physiological changes during nitrogen deprivation. J Appl Phycol 30:3131–3151. https://doi.org/10.1007/s10811-018-1504-4
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81(4):629–636. https://doi.org/10.1007/s00253-008-1681-1
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa. Russ J Plant Physiol 55(4):455–462. https://doi.org/10.1134/S1021443708040043
Gouveia L (2014) From tiny microalgae to huge biorefineries. Oceanography 2(1):1–8. https://doi.org/10.4172/2332-2632.1000120
Khan MI, Lee MG, Shin JH, Kim JD (2017) Pretreatment optimization of the biomass of Microcystis aeruginosa for efficient bioethanol production. AMB Express 7:19. https://doi.org/10.1186/s13568-016-0320-y
Seon G, Kim HS, Cho JM, Kim M, Park WK, Chang YK (2020) Effect of post-treatment process of microalgal hydrolysate on bioethanol production. Sci Rep 10(1):1–2. https://doi.org/10.1038/s41598-020-73816-4
Ho SH, Huang SW, Chen CY, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Kim KH, Choi IS, Kim HM, Wi SG, Bae HJ (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour Technol 153:47–54
Nguyen MT, Choi SP, Lee JW, Lee JH, Sim SJ (2009) Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol 19(2):161–166
Wang X, Liu X, Wang G (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol 53(3):246–252
Acknowledgements
Authors are thankful to the Head, Department of Renewable Energy Engineering, Punjab Agricultural University, Ludhiana, for providing the necessary facilities to carry out this study.
Author information
Authors and Affiliations
Contributions
MST and AK conceived and designed the experimental study. MS was involved in the experimental setup. APK performed the experiments and collected data. The manuscript was written and edited by APK, MST, and AK.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Authorship Consent
All the authors read, approved the final manuscript, and agreed for this submission.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
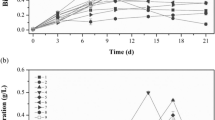
Growth (a.u.) of C. sorokiniana grown in a medium containing different potassium nitrate levels. Values are mean ± standard error of three replicates (DOCX 18 KB)
Fig. S2
Fresh biomass and dry biomass (mg/L) of C. sorokiniana grown in a medium containing different potassium nitrate levels. Values are mean ± standard error of three replicates (DOCX 21 KB)
Rights and permissions
About this article
Cite this article
Kaur, A., Taggar, M.S., Kalia, A. et al. Nitrate-Induced Carbohydrate Accumulation in Chlorella sorokiniana and its Potential for Ethanol Production. Bioenerg. Res. 15, 253–263 (2022). https://doi.org/10.1007/s12155-021-10292-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10292-2