Abstract
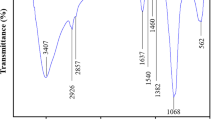
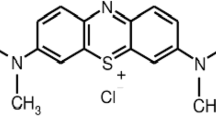
A chitosan polymer was magnetized by coating with magnetite Fe2O3 nanoparticles, and the resultant material (C-Fe2O3) was first characterized through scanning electron microscopy equipped with energy-dispersive X-ray spectroscopy, transmission electron microscopy, atomic force microscopy, thermogravimetric, X-ray diffractometry, Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller, and point of zero charge analyses. C-Fe2O3 was then employed as a separable and efficient adsorptive agent to remove acid blue 113 (AB113) dye from aqueous solution. The removal efficiency was optimized at different environmental parameter values (pH 3–11, C-Fe2O3 dose: 0.1–1 g/L, initial AB113 dye concentration: 10–100 mg/L, adsorption time: 0–300 min, and temperature: 15–45 °C). Under optimum conditions, an AB113 dye removal efficiency of 99.68% was achieved. In addition, the effect of the presence of NaCl, NaNO3, Na2CO3, and MgSO4 ions on the AB113 dye removal efficiency could be ranked as NaCl > NaNO3 > MgSO4 > Na2CO3. The statistical analysis using the coefficient of determination, root mean square error, chi-square test, sum of squared errors, and average relative error showed that the Freundlich and pseudo-second-order equations were the best mathematical models for fitting the isothermal and kinetics data. Further kinetics analyses showed that the adsorption of AB113 molecules on C-Fe2O3 active sites was dominated by the intraparticle diffusion process. Thermodynamic parameters indicated that the AB113 dye adsorption process was favorable, endothermic, and spontaneous. Furthermore, an increase in temperature had a positive impact on AB113 dye removal. The regeneration study confirmed the excellent shelf life of C-Fe2O3, with only a slight loss in the removal efficiency (< 7%) being detected after six operational cycles of AB113 dye adsorption. Compared with other adsorbents, C-Fe2O3 was more effective for the adsorption of AB113 dye, with an adsorption uptake up to 128 mg/g.
Graphic Abstract









Similar content being viewed by others
References
Wu P, Wu T, He W, Sun L, Li Y (2013) Adsorption properties of dodecylsulfat-eintercalated layered double hydroxide for various dyes in water. Colloids Surf A 436:726–731
Samarghandi MR, Tari K, Shabanloo A, MehdiSalari M, Zolghadr NH (2020) Synergistic degradation of acid blue 113 dye in a thermally activated persulfate (TAP)/ZnO-GAC oxidation system: degradation pathway and application for real textile wastewater. Sep Purif Technol 247:116931
Shan R, Yan L, Yang Y, Yang K, Yu S, Yu H (2015) Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J Ind Eng Chem 21:561–568
Bazrafshan E, Ahmadabadi A, Mahvi AH (2013) Reactive Red-120 removal by activated carbon obtained from cumin herb wastes. Fresen Environ Bull 22:584–590
Giustetto R, Wahyudi O (2011) Sorption of red dyes on palygorskite: synthesis and stability of red/purple Mayan nanocomposites. Microporous Mesoporous Mater 142:221–235
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Balarak D, Abasizdeh H, Jalalzayi Z, Rajiv P, Vanathi P (2020) Batch adsorption of acid blue 113 dye from aqueous solution using surfactant-modified zeolite. Indian J Environ Prot 40(9):927–933
Savic I, Gajic D, Stojiljkovic S, Savic I (2014) Modeling and optimization of methylene blue adsorption from aqueous solution using bentonite clay. Comput Aided Chem Eng 33:1417–1422
Zhang YJ, Liu LC, Ni LL, Wang BL (2013) A facile and low-cost synthesis of granulated blast furnace slag-based cementitious material coupled with Fe2O3 catalyst for treatment of dye wastewater. Appl Catal B 138–139:9–16
Balarak D, Al-Musawi TJ, Mohammed IA, Abasizadeh H (2020) The eradication of reactive black 5 dye liquid wastes using Azolla filiculoides aquatic fern as a good and an economical biosorption agent. SN Appl Sci 2(6):1015
Asgari G, Shabanloo A, Salari M, Eslami F (2020) Sonophotocatalytic treatment of AB113 dye and real textile wastewater using ZnO/persulfate: Modeling by response surface methodology and artificial neural network. Environ Res 184:109367
Eren E (2010) Adsorption performance and mechanism in binding of azo dye by raw bentonite. Clean Soil Air Water 38:758–763
Sanghi R, Verma P (2013) Decolorisation of aqueous dye solutions by low-cost adsorbents: a review. Color Technol 129:85–108
Ma J, Cui B, Dai J, Li D (2011) Mechanism of adsorption of anionic dye from aqueous solutions onto organobentonite. J Hazard Mater 186:1758–1765
Li Q, Yue QY, Su Y, Gao BY, Sun HJ (2010) Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem Eng J 158:489–497
Hao Y, Yan L, Yu H, Yang K, Yu S, Shan RL (2014) Comparative study on adsorption of basic and acid dyes by hydroxy-aluminum pillared bentonite. J Mol Liq 199:202–207
Balarak D, Abasizadeh H, Yang JK, Shim MJ, Lee SM (2020) Biosorption of acid orange 7 (AO7) dye by canola waste: equilibrium, kinetic and thermodynamics studies. Desal Water Treat 190:331–339
Elgin AB, Özdemir O, Turan M, Turan AZ (2008) Color removal from textile dye bath effluents in a zeolite fixed bed reactor: determination of optimum process conditions using Taguchi method. J Hazard Mater 159:348–353
Sillanpää M, Mahvi AH (2021) Adsorption of Acid orange 7 dyes from aqueous solution using Polypyrrole/nanosilica composite: experimental and modelling. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1855338
Balarak D, Azarpira H (2016) Biosorption of Acid Orang 7 using dried Cyperus Rotundus: isotherm studies and error functions. Int J ChemTech Res 9:543–549
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Xua L, Wang J (2012) Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl Catal B 123–124:117–126
Kyzas GZ, Deliyanni EA (2013) Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules 18:6193–6214
Bouatay F, Meksi N, Adeel S, Salah F, Mhenni F (2016) dyeing behavior of the cellulosic and jute fibers with cationic dyes: process development and optimization using statistical analysis. J Nat Fiber 13:423–436
Mittal H, Mishra SB (2014) Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydr Polym 101:1255–1264
Kavitha AL, Prabu HG, Babu SA (2012) Synthesis of low-cost Iron Oxide-Chitosan nanocomposite for antibacterial activity. Int J Polym Mater Polym Biomat 62:45–49
Tabak A, Baltas N, Afsin B, Emirik M, Caglar B, Eren E (2010) Adsorption of Reactive Red 120 from aqueous solutions by cetylpyridinium-bentonite. J Chem Technol Biotechnol 85:1199–1207
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021
Khandanlou R, Ahmad MB, Shameli K, Kalantari K (2013) Synthesis and characterization of Rice straw/Fe3O4 nanocomposite by a quick precipitation method. Molecules 18:6597–6607
Sun X, Peng B, Ji Y, Chen J, Li D (2009) Chitosan(chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AlChE 55:2062–2069
Chokami MK, Babaei L, Zamani AA, Parizanganeh AH, Piri F (2017) Synthesized chitosan/iron oxide nanocomposite and shrimp shell in removal of nickel, cadmium and lead from aqueous solution. Glob J Environ Sci Manage 3(3):267–278
Broujeni B, Nilchi A, Hassani AH, Saberi R (2018) Preparation and characterization of chitosan/Fe2O3 nano composite for the adsorption of thorium (IV) ion from aqueous solution. Water Sci Technol 78:708–720
Rahmani A, Salari M, Tari K, Shabanloo A, Shabanloo N, Bajalan S (2020) Enhanced degradation of furfural by heat-activated persulfate/nZVI-rGO oxidation system: degradation pathway and improving the biodegradability of oil refinery wastewater. J Environ Chem Eng 8:104468
Rahmani A, Mohammadi AM, Leili M, Shabanloo A, Ansari A, Alizadeh S, Nematollahi D (2021) Electrocatalytic degradation of diuron herbicide using three-dimensional carbon felt/β-PbO2 anode as a highly porous electrode: influencing factors and degradation mechanisms. Chemosphere 276:130141
Khodadadi M, Al-Musawi TJ, Kamranifar M, Saghi MH, Panahi AH (2019) A comparative study of using barberry stem powder and ash as adsorbents for adsorption of humic acid. Environ Sci Pollut Res 26:26159–26169
Alwared AI, Al-Musawi TJ, Muhaisn LF, Mohammed AA (2021) The biosorption of reactive red dye onto orange peel waste: a study on the isotherm and kinetic processes and sensitivity analysis using the artificial neural network approach. Environ Sci Pollut Res 28(3):2848–2859
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH (2016) Kinetic, isotherms and thermodynamic modeling for adsorption of acid blue 92 from aqueous solution by modified azolla filicoloides. Fresen Environ Bull 25(5):1321–1330
Hu Z, Chen H, Ji F (2015) Removal of Congo red from aqueous solution by cattail root. J Hazard Mater 173:292–297
Fontana KB, Chaves ES, Sanchez JDS, Lenzi GG (2016) Textile dye removal from aqueous solutions by malt bagasse: isotherm, kinetic and thermodynamic studies. Ecotoxicol Environ Saf 124:329–336
Hilal NM, Ahmed IA, Badr EE (2012) Removal of acid dye (AR37) by adsorption onto potatoes and egg husk: a comparative study. J American Sci 8:341–348
Rahmani AR, Salari M, Shabanloo A, Shabanloo N, Bajalan S, Vaziri Y (2020) Sono-catalytic activation of persulfate by nZVI-reduced graphene oxide for degradation of nonylphenol in aqueous solution: process optimization, synergistic effect and degradation pathway. J Environ Chem Eng 8:104202
Shabanloo M, Salari M, Shabanloo N, Dehghanib MH, Pittman CU, Mohane D (2020) Heterogeneous persulfate activation by nano-sized Mn3O4 to degrade furfural from wastewater. J. Mol. Liq. 298:112088
Nasseh N, Khosravi R, Rumman GA, Ghadirian M, Eslami H, Khoshnamvand M, Al-Musawi TJ, Khosravi A (2021) Adsorption of Cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation. Environ Technol Innov 21:101277
Al-Musawi TJ, Brouers F, Zarrabi M (2018) What can the use of well-defined statistical functions of pollutants sorption kinetics teach us? A case study of cyanide sorption onto LTA zeolite nanoparticles. Environ Technol Innov 10:46–54
Rostamian R, Behnejad H (2016) A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf Environ Prot 102:20–29
Samarghandi M, Al-Musawi T, Mohseni-Bandpi A, Zarrabi M (2015) Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J Mo Liq 211:431–441
Balarak D, Mostafapour FK (2018) Adsorption of acid red 66 dye from aqueous solution by heat-treated rice husk. Res J Chem Environ 22(12):80–84
Dotto GL, Pinto LAA (2011) Adsorption of food dyes acid blue 9 and yellow 3 onto chitosan: stirring rate effect in kinetics and mechanism. J Hazard Mater 187:164–170
Yao Y, Xu F, Chen M, Xu Z, Zhu Z (2010) Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol 101:3040–3046
Joshi S, Garg V, Kataria N, Kadirvelu K (2019) Applications of Fe3O@ AC nanoparticle for dye removal from simulated wastewater. Chemosphere. 236:124280
Madrakian T, Afkhami A, Ahmadi M, Bagheri H (2011) Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J Hazard Mater 196:109–114
Balarak D, Mostafapour FK, Joghataei A (2016) Adsorption of: Acid Blue 225 dye by Multi Walled Carbon Nanotubes: Determination of equilibrium and kinetics parameters. Pharm Chem 8:138–145
Mustafa YA, Jaid GM, Alwared AI, Ebrahim M (2014) The use of artificial neural network (ANN) for the prediction and simulation of oil degradation in wastewater by AOP. Environ Sci Pollut Res 21:7530–7537
Fiorentin LD, Trigueros DEG, Pereira NC, Barros STD, Santos OAA (2010) Biosorption of reactive blue 5G dye onto drying orange bagasse in batch system: kinetic and equilibrium modeling. Chem Eng J 163:68–77
Somasekhara R, Sivaramakrishna MC, Varada R (2012) The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J Hazard Mater 203–204:118–127
Mohammed AA, Najim AA, Al-Musawi TJ, Alwared AI (2019) Adsorptive performance of a mixture of three nonliving algae classes for nickel remediation in synthesized wastewater. J Environ Health Sci Eng 17:529–538
Akar T, Celik S, Akar ST (2010) Biosorption performance of surface modified biomass obtained from Pyracantha coccinea for the decolorization of dye contaminated solutions. Chem Eng J 160:466–472
Arami M, Limaee NY, Mahmoodi NM, Tabrizi NS (2006) Equilibrium and kinetics studies for the adsorption of direct and acid dyes from aqueous solution by soy meal hull. J Hazard Mater 135:171–179
Ferrero F (2007) Dye removal by low cost adsorbents: hazelnut shells in comparison with wood sawdust. J Hazard Mater 142:144–152
Lakshmi UR, Srivastava VC, Mall ID, Lataye DH (2009) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics for Indigo Carmine dye. J Environ Manag 90:710–720
Zazouli MA, Ebrahimi CJY, M, Mahdavi Y. (2013) Investigating the removal rate of acid blue 113 from aqueous solution by canola (Brassica Napus). J Mazand Univ Med Sci 22:70–78
Hameed BH, Hakimi H (2008) Utilization of durian (Durio zibethinus Murray) peel as low cost sorbent for the removal of acid dye from aqueous solutions. Biochem Eng J 39:338–343
Akar T, Ozcan AS, Tunali S, Ozcan A (2008) Biosorption of a textile dye (Acid Blue 40) by cone biomass of Thuja orientalis: estimation of equilibrium, thermodynamic and kinetic parameters. Bioresour Technol 99:3057–3065
Meziti C, Boukerroui A (2012) Removal of a Basic Textile Dye from Aqueous Solution by Adsorption on Regenerated Clay. Procedia Engineering 33:303–312
Chu HC, Lin LH, Liu HJ, Chen KM (2013) Utilization of dried activated sludge for the removal of basic dye from aqueous solution. Desal Wat Treat 51:7074–7080
Han R, Zhang J, Han P, Wang Y (2009) Study of equlibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Gok O, Ozcan AS, Ozcan A (2010) Adsorption behavior of a textile dye of Reactive Blue 19 from aqueous solutions onto modified bentonite. Appl Surf Sci 256:5439–5443
Suna D, Zhanga X, Wub Y, Liu X (2010) Adsorption of anionic dyes from aqueous solution on fly ash. J Hazard Mater 181:335–342
Osma JF, Saravia V, Toca-Herrera JL, Couto SR (2007) Sunflower seed shells: a novel and effective low-cost adsorbent for the removal of the diazo dye Reactive Black 5 from aqueous solutions. J Hazard Mater 147:900–905
Acknowledgements
The authors are grateful to the Zahedan University of Medical Sciences (Iran) for the laboratory assistance and financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Musawi, T.J., Mengelizadeh, N., Al Rawi, O. et al. Capacity and Modeling of Acid Blue 113 Dye Adsorption onto Chitosan Magnetized by Fe2O3 Nanoparticles. J Polym Environ 30, 344–359 (2022). https://doi.org/10.1007/s10924-021-02200-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02200-8