Abstract
Mining activities generate much wastes which degenerate into various geochemical components and affect the natural composition of surface water resources. They cause Acid Mine Drainage (AMDs) and influence the hydrochemical evolution of the hydrogeological systems. This study employed hydrochemical parameters to characterize and evaluate the effects of mine discharges on irrigation surface water in the mining district of Enyigba, SE Nigeria. Twenty-four water samples were collected from surface water sources used for subsistence irrigation farming and analyzed for pH, total dissolved solids (TDS), electrical conductivity (EC), sodium (Na+), potassium (K), calcium (Ca2+), magnesium (Mg2+), bicarbonate (HCO3−), chloride (Cl−) and sulfate (SO42−), while various irrigation parameters including Soluble Sodium Percentage (SSP), Magnesium Adsorption Ratio (MAR), Sodium Percentage (Na %), Sodium Adsorption Ratio (SAR), Permeability Index (PI), Total Hardness (TH), Kelly Ratio (KR) and Electrical Conductivity (EC) were deduced. Result indicates hydrogeochemical trend of Cl− > Mg2+ > Ca2+ > SO42− > HCO3− + CO3 > Na+ + K+, while hydrogeochemical facies from Piper Trilinear plot, Durov and Schoeller plots shows that the dominant ionic species are the Mg2+, Cl−, and SO42−. Irrigation parameters such as SSP, MAR, Na %, SAR, PI, KR and EC indicate that majority of water sample are very good to moderately suitable for irrigation. Five samples around Amorie and Ameka are within the hard category for TH, which could be attributed to the high concentration of dissolved magnesium and calcium ion in the area. Apart from mine discharges, rock water interaction also affects the composition of surface water resources of the area.
Similar content being viewed by others
Introduction
In the past few decades, hydrogeological researches have shifted from the occurrence, to the quality of water for domestic, agricultural and industrial uses. This is due to the high demand for quality water. Reddy et al. (2013) noted that the high demand is due to the geometric increase in population, urban development, industrialization and new investments in agricultural production in many parts of the world. Therefore, overexploitation of surface water resources is inevitable. Rawat et al (2018) and Narsimha and Sudarshan (2018) observed that in the semiarid region of Varanasi, Uttar Pradesh and Basara, South India, respectively, the shortage of surface water has led to dependence of groundwater resources in the area for domestic and agricultural purposes. Sridharan and Senthil (2017) also noted that the available quantity of freshwater resources for drinking and irrigation purpose is not enough on the earth’s surface. Anthropogenic and geogenic sources can affect surface and groundwater chemistry and increase pollution status of arable soils and sediments with the subsequent increase of heavy metals in water and soils (Obasi 2017, 2020, 2019b, 2017; Obasi and Akudinobi 2019a; Igwe et al. 2014). The high rate of population growth and industrial development and technological advancement has raised some serious socioenvironmental concerns in water resource management (Singh et al. 2020). Hence, the knowledge of groundwater and surface water hydrochemistry is important to assess the quality of freshwater especially in rural areas as this has serious influence on the suitability of surface and groundwater for domestic, irrigation, and industrial needs.
Irrigation agriculture is necessary for sustainable agricultural development. This is due to the role of irrigation water in crop production. Talukder et al. 1998 emphasized that poor irrigation water may affect crop yield and soil physical conditions. Irrigation water is evaluated by four major important parameters including salinity hazard, sodium hazard, toxicity hazard and sodium carbonate hazard (Michael 1978). Other parameters include Soluble Sodium Percentage (SSP), Magnesium Adsorption Ratio (MAR), Sodium Percentage (Na %), Sodium Adsorption Ratio (SAR), Permeability Index (PI), Total Hardness (TH), Kelly Ratio (KR) and Electrical Conductivity (EC). Groundwater quality studies for drinking and irrigation purposes have been carried out in different parts of the world including Tamil Nadu, India (Ibraheen and Nazeeb Khan 2017; Subramani et al. 2005), Uttarakhand (Jain et al. 2010), Peninsular (Rawat et al. 2018), Punjab (Kumar et al. 2007), Sri Lanka (Nishanthiny et al. 2010), Iran (Aghazadeh and Mogaddam 2010; Narany et al. 2015), Ghana (Ackah et al. 2011), Nigeria (Igwe et al. 2014, 2015, 2017; Eyankware et al. 2016a, 2016b, 2017, 2018, 2020), and China (Wen et al. 2005). These studies have shown variously, the characteristic chemical composition of water resources in different areas as it affects plants and soil properties in agricultural development. Michael (1978), Rawat et al. (2018), Ibraheem and Nazeeb Khan (2017), Brindha and Elango (2011) all noted that studies on agricultural irrigational water has improved crop production and agricultural sustainability in India.
The study area
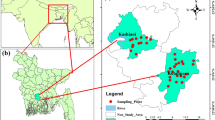
The Enyigba mining district of Ebonyi State southeast Nigeria is richly endowed with abundant lead- zinc and associated mineral (Kogbe 1976; Obage 2009; Igwe et al. 2014, 2015). The mining of this mineral by mechanized and artesian miners enhances the adverse effects of mining activities on water quality, soil and the total biotic environment (Obasi and Akudinobi 2015; Obasi 2017; Obasi et al. 2018; Igwe et al. 2014, 2017). Mining wastes (tailing) are been channeled into surface and farmlands (Fig. 1), thereby degrading the quality of water for domestic, irrigation and other uses (Obasi 2017). Several works including Obiorah et al. (2016, 2018), Obasi and Akudinobi 2019a, 2019b, 2020), Obasi and Akudinobi (2015), Nnabo et al. (2011), Igwe et al. (2012, 2014, 2015, 2017), Oti and Nwabue (2013) and Ezeh et al. (2007) have been done with regard to water quality, food crops and soil contamination in the Enyigba area. But none of these has established the quality of these water resources with respect to irrigation agriculture which is leading the economic trend of a diversified economy in Nigeria. Irrigation agriculture is dependent on water supply of good quality (Eyankware et al. 2018; Rawat et al. 2018). Water of poor quality is not suitable for neither human nor plant uses. Nata et al. (2011) noted that water from mining activities cannot be satisfactorily used for irrigation purposes.
The present agricultural policy of both the Ebonyi State and Federal Government of Nigeria makes this study very apt and necessary. This is because the Enyigba area in Abakaliki is one of the major plains of Ebonyi State where the famous “Abakaliki Rice” is cultivated. World Bank Assisted projects like FADAMA and International Fund for Agricultural Development (IFAD) projects have been seriously embraced by the people of the area. It is against this background that an evaluation of the surface water resources is necessary. This study will characterize and evaluate the effects of mine discharge on the surface water resources for irrigation purposes. Water quality characteristics for irrigation including Soluble Sodium Percentage (SSP), Magnesium Adsorption Ratio (MAR), Sodium Percentage (Na %), Sodium Adsorption Ratio (SAR), Permeability Index (PI), Total Hardness (TH), Kelly Ratio (KR) and Electrical Conductivity (EC) will be determined. This will enhance the full agricultural participation of the people of the area into irrigation farming, especially in the dry season where there is shortage of rain water.
Geology and physiography
The Benue Trough is an elongate intracontinental Cretaceous basin (about 1000 km in length), stretching in a NE-SW direction and resting unconformably upon the Precambrian Basement rocks (Burke et al. 1972; Nwachukwu 1975). It extends from the Gulf of Guinea to the Chad basin and is thought to have been formed by the “Y”-Shaped triple—R Junction ridge system that initiated the breaking up and separation of the Afro-Brazilian plates in the Early Cretaceous, Burke et al. (1972) (Fig. 2). The basin is renowned geologically, because of the occurrence of solid minerals in the sediments. The stratigraphic and tectonic history has been widely studied (Reyment 1969; Benkhelil 1989). And three sedimentary phases/cycles have been described in the regional stratigraphic history of southeastern Nigeria (Short and Stauble 1967; Murat 1972). These three phases include the Abakaliki-Benue phase/the first sedimentary cycle (Aptian-Santonian); the Anambra-Benin phases/second sedimentary cycle (Campanian—Mid Eocene); and the Niger Delta phase/third sedimentary cycle (late Eocene-Pliocene). The study area belongs to the first phase.
Locally, the geology of the Enyigba area is that of the slightly deformed Cretaceous sedimentary rocks made up of essentially thick marine dark-gray Albian Shales (Abakaliki Shales) and mudstones of the Asu River Group (ARG), and occasional bands of ironstone and lenses of sandy limestones. The shales are fissile with characteristic bluish-gray and reddish-brown coloration (Kogbe 1976). The Ezeaku Shales (EZS) are also present at the Southeast corner of the area where the boundary between the two geologic formations has been inferred (see Fig. 2). Generally, the environment has witnessed the regional stress of the Santonian orogeny which had led to the folding, fracturing and mineralization of almost the whole environment (Nwachukwu 1975; Olade 1976). The mineralization described by Farrington (1952) as hydrothermal in origin, deposited under mesothermal conditions and related to intrusive activity consists predominantly of galena and sphalerite and occurs entirely within fracture zones associated with the dark carbonaceous shales, the host rocks. The ore minerals (galena) are associated with a variety of gangue minerals such as siderite, calcite, quartz and barites (Farrington 1952). The trends of joints, veins/veinlets and sulfide lodes in the area occur mainly in the NE-SW orientation (Obasi 2017).
The climate of the area is characterized by two seasons, viz. the dry and the rainy seasons. The rainy season begins in March and ends in October while the dry season begins in October and ends in February. Temperature in the dry season ranges from 25 to 29 °C and during the rainy season, 16 °C to 28 °C (Obasi and Akudinobi 2015). The average annual rainfall varies from 1750 to 2250 mm. This high amount of rainfall results in surface run-off which transports the pollutants offsite and also facilitates dispersion, infiltration and percolation with ease. The study area is part of the rainforest region of southeastern Nigeria. It has a humid climate and evergreen vegetation. The vegetation cover is composed of very dense trees and underground of creepers. These trees are mostly tall with buttress roots. The vegetation is controlled by many factors, including the drainage, topography, geology and rainfall. The area has been described as part of the low land rainforest region (Iloeje 1979).
Materials and methods
Sample Collection and Laboratory Analysis
Twenty-four water samples were collected from surface water sources used for irrigation; these sources are receptacles for mine discharges in the study area (Fig. 2). The samples were analyzed for physical and geochemical qualities using standard methods. These samples were collected in the dry season for optimum concentration of hydrochemical species. Prior to collection, the bottles were washed with clean water and carefully rinsed with distilled, de-ionized water and later soaked in distilled water acidified with 1.0 mL of HNO3 for three days. Electrical Conductivity (EC), pH and Total Dissolved Solids (TDS) were determined at points of collection. Samples were corked and stored in ice chests polyethylene bottles and transported to the laboratory within 24 hours of collection for analysis of physicochemical properties. Electrical conductivity and total dissolved solids were determined using the HACH conductivity and TDS meters (model HQ14D53000000, USA). pH meter HachsensION + PH1 portable pH meter and HachsensION + 5050 T Portable Combination pH Electrode were used to measure pH. Potassium (K+) and Sodium (Na+) ion concentrations were obtained with a Jenway Clinical flame photometer (PFP7 model). Calcium (Ca), Magnesium (Mg) and Chloride (Cl−) ions were determined using appropriate titrimetric methods according to APHA (2012), while sulfate concentration was determined by turbidimetric method using a UV–Vis spectrometer and spectra manager software for interpretation. Ionic balance within (1:1 ± 0.01%) was calculated to determine the accuracy of geochemical analysis as plotted using Surfer 10 software package. Irrigation parameters were determined by calculating the relations below in (meq/L). The suitability of surface water for irrigation was evaluated by comparing the water samples with various water quality standards for irrigation.
Analytical check/ionic balancing
The accuracy of the geochemical result was assessed using the relationship between the anions and the cations in the analyzed samples as expressed in meq/L. The equations according to Hounslaw (1995), Domenico and Schwartz (1990) are represented as;
The above equation gave a cation–anion ratio of 1:1 ± 0.01, which confirms that the geochemical analysis was accurate. The cation–anion balance was also assessed using electrical neutrality equation which requires that the sum of positive ions must be equal to sum of negative ions in solution expressed in meq/L.
Irrigation parameters
Soluble Sodium Percentage (SSP)
As proposed by Richards (1967) and Todd (1980), it denotes the concentration of sodium in water used for irrigation.
Magnesium Adsorption Ratio (MAR)
Raghunath (1987) assessed the suitability of magnesium ion in natural water using the above parameter. The equation below is used to calculate MAR.
Sodium percentage (Na %)
Sodium percentage is a major parameter in defining suitability of water for irrigation. It is also an efficient tool in the study of sodium hazard. Eaton (1950) and Doneen (1964) studied the effect of high sodium in natural water using the equation below
Sodium Adsorption Ratio (SAR)
Richards (1967) determined the concentration of sodium in relation to Na+, Ca2+, Mg2+ is assessed using the equation according to calculate SAR. Thus,
Kelly’s ratio (KR)
This parameter measures the concentration of Na+ against the concentration of the alkaline earth metals (Ca2+ and Mg2+). The equation by Kelly (1963) is used to calculate KR.
Total Hardness (TH)
The softness or hardness of water for irrigation is assessed using the relation by Sawyer and McCarty 1967; Raghunath 1987). Thus,
Permeability Index (PI)
PI is used to evaluate soil permeability as affected by over exposure of soil to irrigation due to the concentration of sodium, calcium, magnesium and bicarbonate ions in natural water. The equation below according to Domenico and Schwartz (1990) is used to calculate PI.
Results
See Table 1.
Discussion
Irrigation parameters
Electrical Conductivity (EC)
The values of EC in the study area range from 0.17 to 918.00 µS/cm. This parameter is related to the concentration of dissolved salts which imparts in water salinity. From Richards (1967) classification of EC (Table 2), 79.16% of samples analyzed are classified under the excellent category. These are samples OP/SW/1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19 and 21. Hence, these samples are considered fit for irrigation. Sample location OP/SW/20, 22, 23 and 24 (i.e., 16.6%) is also considered fit for irrigation, while sample location OP/SW/17 (i.e., 4.16%) is considered not fit for irrigation. This could be attributed to the presence of mobile ionic species generated from mine operations in the area (Obasi et al. 2018). Reddy et al. (2013) stated that the primary effect of high EC in irrigation water on crop production is the inability of the plant to compete with ions in the soil solution for water. The higher the EC, the lesser the water available for plants; this is because plants can only transpire “pure” water no matter the quantity of water available in the soil. Studies have also shown that the amount of water transpired through a crop is directly proportional to the yield; therefore, irrigation water with high EC reduces potential yield (Datta and Dayal 2000).
Soluble Sodium Percentage (SSP)
According to USDA (1954) when values of SSP < 50 it indicate that water is considered suitable for irrigation, while values > 50 indicates the unacceptability of water for irrigation. The value of SSP within the study area ranges from 0.00 to 100.00. 91.6% of samples were below the set limit. Only samples OP/SW/1 and OP/SW/8 with value of 51.72 and 100.00, respectively, were above the limit (Fig. 3, Tables 3 and 4). There is low concentration of sodic minerals in the shales of the study area (Olade 1976). Excess sodium in water changes soil properties and reduces soil permeability. This is very undesirable in crop production (Biswas et al. 2002).
Wilcox diagram of SSP classification for water sample of the study area (after Wilcox 1955)
Sodium percentage (Na %)
The value of Na % ranges from 0.00 to 120 as shown in Tables 3 and 4. Wilcox diagram (1955) (Fig. 3) showed that all samples were below the excellent to good category except for sample location OP/ SW/12. This implies that sample location OP/SW/1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 (i.e., 95.8%) is considered fit for irrigation, while sample OP/SW/12 is considered doubtful. This could also be attributed to the non-occurrence and subsequent nondissolution of sodic minerals in the area. Veena and Satheeshkumar (2018) noted that a major cause of high sodium percentage in surface water is the additions of chemical fertilizers; this does not apply to the study area. The use of high Na % water for irrigation can lead to stunt growth of plants. Joshi et al. (2009) noted that plants and sodium reacts with soil to reduce soil permeability (Fig. 4).
Rating of water samples on the basis of electrical conductivity and sodium percentage (after Wilcox 1955)
Sodium Adsorption Ratio (SAR)
The concentrations of Na+, Ca2+, and Mg2+ in water samples are studied in detail by SAR (Richards 1967). This is possible because SAR considers the effects of the presence of calcium and magnesium ions on sodium. Veena and Satheeshkumar (2018) further noted that the application of SAR in irrigation studies is necessary because it studies the cation exchange reaction in soil. High values of SAR (above 12 to 15) indicate serious physical soil problems as plants may have difficulty absorbing water (Munshower 1994; Brady and Weil 2002). Reddy et al. (2013) emphasized that high SAR makes the soil compact and impervious. The value of SAR ranges from 0.00 to 0.85, with sample location OP/SW/1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19,20 and 21 classified under the C1 and S1 category hence, excellent and fit for irrigation purpose. Sample locations OP/SW/22, 23 and 24 fall under C2 category (good) and are also fit for irrigation, and finally, sample location OP/SW/18 falls under the doubtful category, therefore considered not fit for irrigation purpose as shown in Fig. 5 and Tables 3 and 4.
Classification of Water based on US salinity diagram (after Richards 1967)
Permeability Index (PI)
PI is very important in the determination of irrigation water as it affects soil permeability and this regulates the movement of water and subsequent intake of nutrients by plants. According to Reddy et al. (2013), PI is a determinant factor in the long-term use of irrigation water. The value of PI ranges from 0.02 to 0.43 with average value of 0.15. Based on value range of PI it is fit for irrigation purpose (Tables 3 and 4). PI is classified under Class I (> 75% permeability), Class II (25–75% permeability) and Class III (< 75% permeability) orders. Sample locations OP/SW/1, 7, 10, 18 and 21 fall within Class 1 category hence it is considered fit for irrigation, while sample locations OP/SW/2, 3, 4, 5, 6, 8, 9, 11,12, 13, 14, 15, 16, 17, 19, 22, 23 and 24 (i.e., 81.8%) fall within the Class II category as shown in Fig. 6.
Doneen’s (1964) Chart for P.I. values of surface water
Total Hardness (TH)
The TH value ranges from 0.00 to 902.50 meq/L (Table 3). Samples OP/ SW/1, 2, 3, 5, 8, 9, 12, 15, 16, 19, 22, 23 and 24 (i.e., 54.1) fall within the soft category, hence fit for irrigation. While sample location OP/SW/04, 14, 17, 20 (i.e., 16.6%) falls within the moderate category, sample location OP/ SW/6 and 13 (8.3%) falls with the hard category and finally sample locations OP/SW/07, 10, 11, 18 and 21 (20.8%) fall within the very hard category; hence, it is considered not suitable for irrigation (Tables 3 and 4). High TH in irrigation water leads to poor plant’s absorption of ions. This affects both the SAR and SPP.
Kelly Ratio (KR)
Singh et al. (2020) stated that the quality of water for irrigation is good if Kelly’s ratio is equal to or below 1, whereas above 1 it is suggestive of unsuitability for agricultural purpose due to alkali hazards. The value of KR ranges from 0.00 to 0.63 (see Table 4). Sample locations OP/SW/1 and 12 are above the set standard for irrigation due to alkali hazard, and such alkali hazard could be introduced from alkaline rocks which occur as minor intrusions in the Enyigba and Imogu areas. However, sample locations OP/SW/2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 and 24 (i.e., 95.8%) are suitable for irrigation uses (see Table 3 and 4).
Hydrochemical facies analysis
The Mg–SO42−–Cl and SO42−–Ca2+ –Mg2+ water types were observed in the area using classifications of the Piper diagram (Fig. 7). The dominant ionic species in the area are Mg2+, Cl−, Ca2+ and SO42−.
Piper trilinear diagram of surface water resources in the Enyigba area (after Piper 1944)
Durov plot (Fig. 8) indicates dominance of Mg2+and SO42−, while Schoeller semilog diagram (Fig. 9) shows the hydrogeochemical trend of Cl− > Mg2+ > Ca2+ > SO42− > HCO3− + CO3 > Na+ + K+. Investigations of hydrochemical facies indicate variations in ionic facies. Cl− and SO42− are the dominant types, with Ca–Cl, Mg–SO42−, Ca–Mg–Cl, Mg–SO42 and Ca–SO42 as minor constituents. This variation in facies indicates the mixing of water from different ground and surface water sources, while chemical processes of ion exchange have been attributed to the increased concentration of Ca2+ and Mg2+ in relation to Na+. Interpretation of Piper diagram using Hem (1991) classification indicates that there is dominance of alkali earths metals in the water resources of the area. Therefore, Ca + Mg and SO4 + Cl facies dominates the water sources of the area. This hydrochemical facies confirms different provenance sources characterized by different lithologic units. The presence of SO42− accompanied by Ca2+ and Mg2+ ions can be attributed to organic shales of marine origin and the presence of calcareous limestones, while Na + and K + ions are from feldspar, which shows the influence of intrusive rocks in the geology of the area.
Durov plot of surface water resources in the Enyigba area (after Hem 1991)
Schoeller diagram of surface water resources in the Enyigba area (after Hem 1991)
Summary and conclusion
Characterization and evaluation of the effects of mine discharges on surface water resources for irrigation uses in the Enyigba mining district, southeast Nigeria, have been carried out in this study. Twenty-four mine receptacles surfaces water samples were collected and analyzed using spectrophotometric methods, while irrigation parameters including Soluble Sodium Percentage (SSP), Magnesium Adsorption Ratio (MAR), Sodium Percentage (Na %), Sodium Adsorption Ratio (SAR), Permeability Index (PI), Total Hardness (TH), Kelly Ratio (KR) and Electrical Conductivity (EC) were deduced. Result of irrigation parameters indicates that majority of water sample (over 85%) ranged from very good to moderately suitable for irrigation. Except for TH where five samples (OP/SW/07, 10, 11, 8 and 21) fall within the hard category. This could be attributed to presence of dissolved magnesium and calcium ion in the water samples. Dominant ionic species of Mg2+, Cl−, and SO42− with hydrogeochemical trend of Cl− > Mg2+ > Ca2+ > SO42− > HCO3− + CO3 > Na+ + K+ were obtained from the surface water plots on Piper diagram, Durov and Schoeller. Surface water resources in the area can be used for irrigation to drive the agricultural programs in the area.
References
Ackah M, Agyemang O, Anim AK, Osei BNO, Kpattah L, Hanson JEK (2011) Assessment of groundwater quality for drinking and irrigation: the case study of Teiman-Oyarifa Community, GaEast Municipality, Ghana. Proc Int Acad Ecol Environ Sci 1(3–4):186–194
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area, Northwest of Iran. J Environ Prot 1(01):30
America Public Health Association (APHA) (2012) Standards methods for the examination of water and wastewater, 22ndedn. America Public Health Association (APHA), America Water Works Association (AWWA) and Environmental Federation (WEF), New York, p 1360
Benkhelil J (1989) Cretaceous deformation, magmatism and metamorphism in the lower benue trough. Nigeria in African geology reviews. J Min 20(10):56–90
Biswas SN, Mohabey H, Malik ML (2002) Assessment of the irrigation water quality of river Ganga in Haridwar District. Asian J Chem 16:285–292
Brady NC, Weil RR (2002) The nature and properties of soils. Prentice-Hall, New Jersey
Brindha K, Elango L (2011) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Madhuranthakan Tamil Nadu, India. Earth Sci Res J 15(2):101–108
Burke KC, Dessawvagia RFJ, Whiteman AW (1972) Geology history of the Benue Valley and adjacent area, Africa Geology, University of Ibadan Press. Geology 10(5):187–206
Datta KK, Dayal B (2000) Irrigation with poor quality water: an empirical study of input use, economic loss and cropping strategies. Indian J Agric Econ 55(1):26–37
Domenico PA, Schwartz FW (1990) Physical and chemical hydrology. Wiley, New York, p 410
Doneen LD (1964) Water quality for agriculture. Department of Irrigation, University of California, Davis, p 48
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Eyankware MO (2016) Hydrochemical appraisal of groundwater for irrigation purpose: a case study of Ekaeru Inyimagu and Its Adjoining Area, Ebonyi State, Nigeria. Indian J Sci 23(88):924–943
Eyankware MO, Obasi PN, Akakuru OC (2016) Use of hydrochemical approach in evaluation of water quality around the vicinity of Mkpuma Ekwaoku Mining District, Ebonyi State, SE. Nigeria for irrigation purpose. Indian J Sci 23(88):881–895
Eyankware MO, Selemo AOI, Omo-Irabor OO (2017) Hydrogeochemical evaluation and suitability study of groundwater for domestic and irrigation purpose. A case study of Eruemukohwarien Community, Niger Delta Region, Nigeria. Sci Technol 3(10):91–108
Eyankware MO, Nnajieze VS, Aleke CG (2018) Geochemical assessment of water quality for irrigation in abandoned limestone quarry pits at Nkalagu Area, Southern Benue Trough Nigeria. Environ Earth Sci 77:66. https://doi.org/10.1007/s12665-0187232-x
Eyankware MO, Obasi PN, Omo-Irabor OO, Akakuru OC (2020) Hydrochemical characterization of abandoned quarry and mine waters for domestic and irrigation uses in Abakaliki, Southeast Nigeria. Model Earth Syst Environ. https://doi.org/10.1007/s40808-020-00827-5
Ezeh HN, Anike OL, Egboka BCE (2007) The distribution of some heavy metals in soil in areas around the Derelict Enyigba mines and its environmental implication. Journ Min Geol 2(2):99–106
Farrington JL (1952) A preliminary describtion of the Nigerian lead–zinc field. Econ Geol 47(5):583–608
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water: USGS professional paper book 2254. Scientifc Publishers, Jodhpur
Hounslaw W (1995) Water quality data: analysis and interpretation. CRC Press, Boca Raton, pp 71–127
Ibraheen AM, Khan N (2017) Suitability assessment of groundwater for irrigation purpose in Veppanthattai block, Perambalur District, Tamil Nadu, India. WSN 81(2):81–93
Igwe O, Adepehin EI, Iwuanyanwu C (2012) Environmental effects of the mining of lead zinc minerals in Enyigba and its suburbs, Southern Benue Trough, Nigeria. Niger J Educ Heath Technol Res 3(2):30–44
Igwe O, Adepehin EJ, Iwuanyanwu C, Una CO (2014) Risks associated with the mining of Pb–Zn minerals in some parts of the Southern Benue Trough, Nigeria. Environ Monit Assess 186(6):3755–3765
Igwe O, Adepehin EJ, Adepehin JO (2015) Integrated geochemical and microbiological approach to water quality assessment: case study of the Enyigba metallogenic province, South-eastern Nigeria. Environ Earth Sci 74(4):3251–3262
Igwe O, Una CO, Abu E, Adepehin EJ (2017) Environmental risk assessment of lead–zinc mining: a case study of Adudu metallogenic province, middle Benue Trough, Nigeria. Environ Monit Assess 189(10):492
Iloeje NP (1979) A new geography of Nigeria. Revised New Edition. pp 32–45
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess 166(1–4):663–676
Joshi DM, Kumar A, Agrawal N (2009) Assessment of the irrigation water quality of river Ganga in Haridwar District India. J Chem 2(2):285–292
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(4):355–439
Kogbe CA (1976) Palegeographic history of Nigeria from Albian times. In: Kogbe CA (ed) Geology of Nigeria. Elizabeth Publishers, Lagos, pp 237–252
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53(3):553–574
Michael AM (1978) Irrigation theory and practice. Vikas Publishing House Pvt. Ltd, New Delhi, pp 713–713
Munshower FF (1994) Practical handbook of disturbed land re-vegetation. Lewis Publishers, Boca Raton
Murat RC (1972) Stratigraphy and paleogeography of the cretaceous and lower tertiary in Southern Nigeria. In: Dessauvagie TFJ, Whiteman AJ (eds) African geology. Ibadan University Press, Ibadan, pp 251–266
Narsimha A, Sudarshan V (2018) (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Narany TS, Ramli MF, Aris AZ, Sulaiman WNA, Fakharian K (2015) Groundwater irrigation quality mapping using geostatisticaltechniques in Amol-Babol Plain. Iran Arab J Geosci 2:961–976
Nata T, Abraham B, Bheemalingeswara K, G/yohannes T (2011) Suitability of groundwater quality for irrigation with reference to hand dug wells, Hantebet Catchment, Tigray, Northern Ethiopia. CNCS Mekelle Univ 3(2):31–47
Nishanthiny SC, Thushyanthy M, Barathithasan T, Saravanan S (2010) Irrigation water quality based on hydrochemical analysis, Jaffna, Sri Lanka. Am Eur J Agric Environ Sci 7(1):100–102
Nnabo PN, Orazulike DM, Offor CO (2011) The preliminary assessment of the level of heavy elements contaminations in stream bed sediments of Enyigba and Environs, Southeastern Nigeria. J Basic Phys Res 2(2):43–52
Nwachukwu SO (1975) Temperature of formation of vein, minerals in the south portion of the Benue Trough, Nigeria. J Min Geol 11(12):4–55
Nwajide CS (2013) Geology of Nigeria’ s sedimentary basins. CSS Bookshops Limited, Lagos, Nigeria
Obage GN (2009) Geology and mineral resources of Nigeria. Springer, New York
Obasi PN, Akudinobi BEB (2015) Geochemical assessment of heavy metal distribution and pollution status in soil/stream sediment in the Ameka Minning Area of Ebonyi State, Nigeria. Afr J Geo-Sci Res 3(4):01–07
Obasi PN (2017) Hydrogeological and geochemical assessment of the lead–zinc mining areas of Abakaliki Ebonyi State, Southeastern Nigeria. Unpublished Ph.D. thesis. Nnamdi Azikiwe University, Awka, Nigeria
Obasi PN, Esom NE, Okolo CM, Edene EN (2018) Assessment of water pollution status in the mining area of Ameka, South Eastern Nigeria using metal pollution index. Int J Sci Eng Sci 2(1):66–73
Obasi PN, Akudinobi BEB (2019a) Pollution status of arable soils and stream sediments in mining areas of Abakaliki, Lower Benue Trough, Nigeria. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02337-z
Obasi PN, Akudinobi BEB (2019b) Heavy metals occurrence, assessment and distribution in water resources in the lead–zinc mining areas of Abakaliki, Southeastern Nigeria. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02489-y
Obasi PN (2020) Occurrence and distribution of heavy metals in arable soils around lead–zinc mining sites of Abakaliki, Southeast Nigeria. Model Earth Syst Environ. https://doi.org/10.1007/s40808-020-00800-2
Obiorah SC, Chukwu A, Toteu SF, Davies TC (2016) Assessment of heavy metals contamination in soils around Pb–Zn mining areas in Enyigba, Southeastern Nigeria. J Geol Soc India 87:453–462
Obiorah SC, Chukwu A, Toteu SF, Davies TC (2018) Contamination of the potable water supply sources in the lead–zinc mining communities of Enyigba, Southeastern Nigeria. Mine Water Environ. https://doi.org/10.1007/s10230-018-0550-0
Olade MA (1976) The genesis of lead-zinc deposits in Nigeria’s Benue rift (aulacogen): a re-interpretation. J Min Geol 13(2):20–27
Oti WJO, Nwabue FI (2013) Heavy metals effect due to contamination of vegetables from Enyigba Lead Mine in Ebonyi State, Nigeria. Environ Pollut 2(1):19–26
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans-Am Geophy Union 25:914–923
Rawat KS, Singh SK, Gautam SK (2018) Assessment of Groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:233. https://doi.org/10.1007/s13201-018-0866-8
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Reddy KJ, Wang L, Gloss SP (2013) Solubility and mobility of copper, zinc and lead in acidic environments. Plant Soil 171:53–58
Reyment RA (1969) Aspects of the geology of Nigeria. University of Ibadan Press, Ibadan, p 144
Richards (1967) Diagnosis and improvement of saline and alkali soils. In: United states salinity laboratory staff agricultural handbook No. 60. The United States Government Printing Office, Washington DC
Sawyer CN, McCarty PL (1967) Chemistry for Sanitary Engineers, 2nd edn. McGraw-Hill, New York, p 518
Singh KK, Tewari G, Kumar S (2020) Evaluation of Groundwater quality for suitability of Irrigation purpose: a case study in the Udham Singh Nagar. Hindawi J Chem, Uttarakhand. https://doi.org/10.1155/2020/6924026
Short KC, Stauble AI (1967) Outline of the geology of Nigeria Delta. Hull AAPG 54:761–779
Sridharan M, Senthil DN (2017) Groundwater quality assessment for domestic and agriculture purposes in Puducherry region. Appl Water Sci 7:4037–4053
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47(8):1099–1110
Talukder MSU, Shirazi SM, Paul UK (1998) Suitability of Groundwater for Irrigation at Kirimganj Upazila Kishoreganj. Prog Agric 9:107–112
Todd DK (1980) Groundwater hydrology. Wiley, New York
USDA (1954) U.S.D.A., Salinity Laboratory Staff, U.S. Department of Agriculture HandBook.60. US Govt. Printing Office, Washington, DC
Veena C, Satheeshkumar S (2018) Assessment of groundwater quality for drinking and irrigation purposes in arid areas of Rajasthan, India. Appl Water Sci 8:218. https://doi.org/10.1007/s13201-018-0865-9
Wen X, Wu Y, Su J, Zhang Y, Liu F (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina Basin, Northwestern China. Environ Geol 48:665–675
Wilcox LV (1955) Classification and use of irrigation water. USDA, Circular, Washington, DC, p 96
Zarboski PM (1997) A review of the cretaceous system in Nigeria, Africa. Geosci Rev 5:385–483
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obasi, P.N., Eyankware, M.O. & Akudinobi, B.E.B. Characterization and evaluation of the effects of mine discharges on surface water resources for irrigation: a case study of the Enyigba Mining District, Southeast Nigeria. Appl Water Sci 11, 112 (2021). https://doi.org/10.1007/s13201-021-01400-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01400-w