Abstract
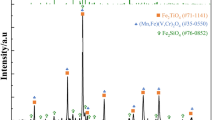
The fusion of the leaching and purification processes was realized by directly using microemulsion as the leaching agent. The bis-(2-ethyhexyl) phosphoric acid (DEHPA)/n-heptane/NaOH microemulsion system was established to directly leach vanadates from sodium-roasted vanadium slag. The effect of the leaching agent on the leaching efficiency was investigated, in addition to the molar ratio of H2O/NaDEHP (W), DEHPA concentration, solid/liquid ratio, stirring time, and leaching temperature. In optimal situations, the vanadium leaching efficiency reaches 79.57%. The X-ray diffraction characterization of the leaching residue and the Raman spectrum of the microemulsion before and after leaching demonstrate the successful entry of vanadates from the sodium-roasted vanadium slag into the microemulsion. The proposed method successfully realizes the leaching and purification of vanadates in one step, thereby greatly reducing production costs and environmental pollution. It also offers a new way to achieve the green recovery of valuable metals from solid resources.
Similar content being viewed by others
References
R.R. Moskalyk and A.M. Alfantazi, Processing of vanadium: a review, Min. Eng., 16(2003), No. 9, p. 793.
D.X. Huang, Re-Vanadium and Steel-Making, The Metallurgy Industry Press, Beijing, 2000, p. 55.
X.H. Li, J. Kou, T.C. Sun, S.C. Wu, and Y.Q. Zhao, Effects of calcium compounds on the carbothermic reduction of vanadium titanomagnetite concentrate, Int. J. Miner. Metall. Mater., 27(2020), No. 3, p. 301.
W.D. Tang, S.T. Yang, and X.X. Xue, Effect of Cr2O3 addition on oxidation induration and reduction swelling behavior of chromium-bearing vanadium titanomagnetite pellets with simulated coke oven gas, Int. J. Miner. Metall. Mater., 26(2019), No. 8, p. 963.
Y.M. Zhang, L.N. Wang, D.S. Chen, W.J. Wang, Y.H. Liu, H.X. Zhao, and T. Qi, A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite, Int. J. Miner. Metall. Mater., 25(2018), No. 2, p. 131.
Z. Wang, H.Y. Sun, and Q.S. Zhu, Effects of the continuous cooling process conditions on the crystallization and liberation characteristics of anosovite in Ti-bearing titanomagnetite smelting slag, Int. J. Miner. Metall. Mater., 26(2019), No. 9, p. 1120.
X.S. Li, B. Xie, G.E. Wang, and X.J. Li, Oxidation process of low-grade vanadium slag in presence of Na2CO3, Trans. Nonferrous Met. Soc. China., 21(2011), No. 8, p. 1860.
Z. Yang, H.Y. Li, X.C. Yin, Z.M. Yan, X.M. Yan, and B. Xie, Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid, Int. J. Miner. Process., 133(2014), p. 105.
B. Liu, H. Du, S.N. Wang, Y. Zhang, S.L. Zheng, L.J. Li, and D.H. Chen, A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3 binary system, AlChE J., 59(2013), No. 2, p. 541.
H.Y. Li, H.X. Fang, K. Wang, W. Zhou, Z. Yang, X.M. Yan, W.S. Ge, Q.W. Li, and B. Xie, Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching, Hydrometallurgy, 156(2015), p. 124.
J. Wen, T. Jiang, Y.J. Liu, and X.X. Xue, Extraction behavior of vanadium and chromium by calcification roasting-acid leaching from high chromium vanadium slag: Optimization using response surface methodology, Miner. Process. Extr. Metall. Rev., 40(2019), No. 1, p. 56.
H.B. Liu, H. Du, D.W. Wang, S.N. Wang, S.L. Zheng, and Y. Zhang, Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method, Trans. Nonferrous Met. Soc. China, 23(2013), No. 5, p. 1489.
Y.M. Zhang, S.X. Bao, T. Liu, T.J. Chen, and J. Huang, The technology of extracting vanadium from stone coal in China: History, current status and future prospects, Hydrometallurgy, 109(2011), No. 1–2, p. 116.
P.G. Ning, X. Lin, H.B. Cao, and Y. Zhang, Selective extraction and deep separation of V(V) and Cr(VI) in the leaching solution of chromium-bearing vanadium slag with primary amine LK-N21, Sep. Purif. Technol., 137(2014), p. 109.
H.Y. Li, C. Li, M. Zhang, K. Wang, and B. Xie, Removal of V(V) from aqueous Cr(VI)-bearing solution using anion exchange resin: Equilibrium and kinetics in batch studies, Hydrometallurgy, 165(2016), p. 381.
Y. Guo, D.Q. Li, B. Xie, and H.Y. Li, Efficient extraction of V(V) in aqueous solution by microemulsion system, [in] The 148th TMS Annual Meeting & Exhibition, San Antonio, 2019, p. 31.
K. Letts and R.A. Mackay, Reactions in microemulsions. I. metal ion incorporation by tetraphenylporphine, Inorg. Chem., 14(1975), No. 12, p. 2990.
M.J. Schwuger, K. Stickdornt, and R. Schomaecker, Microemulsions in technical processes, Chem. Rev., 95(1995), No. 4, p. 849.
Y. Guo, H.Y. Li, X. Zhang, J. Huang, J.K. Feng, J. Diao and, B. Xie, Steering polyoxometalate transformation from octahedral to tetrahedral coordination by counter-cations, Dalton Trans., 49(2020), No. 3, p. 583.
J. Wang, Preparation And Application of Microemulsion, China Textile & Apparel Press, Beijing, 2011, p. 48.
L.M. Prince, Formulation, Microemulsions Theory and Practice, Academic Press, Pittsburgh, 1977, p. 33.
A.Z. Ma, X.J. Cui, G.F. Zeng, X. Cui, L. Tian, H. Xu, and L.M. Li, Infrared and raman spectra of rare earth complexes with bis-(2-ethylhexyl)-phosphoric acid, Chin. J. Spec. Lab., 23(2006), No. 5, p. 893.
F.R. Dorinci, W.G. Fortley, and F.F. Bentley, Characteristic Raman Frequency of Organic Compounds, Chinese Chemical Society, Beijing, 1980, p. 15.
F.D. Hardcastle and I.E. Wachs, Determination of vanadium-oxygen bond distances and bond orders by Raman spectro-scopy, J. Phys. Chem., 95(1991), No. 13, p. 5031.
R.L. Frost and S.J. Palmer, Raman spectroscopic study of pascoite Ca3V10O(28)·17H2O, Spectrochim. Acta, Part A, 78(2011), No. 1, p. 248.
Z.G. Deng, C. Wei, G. Fang, M.T. Li, C.X. Li, and X.B. Li, Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction, Trans. Nonferrous Met. Soc. China., 20(2009), Suppl. 1, p. 118.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51474041 and 51674051), Chongqing Science and Technology Bureau (No. cstc2019jcyjjqX0006), and Chongqing Talents Plan for Young Talents (No. CQYC201905050).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Y., Li, Hy., Yuan, Yh. et al. Microemulsion leaching of vanadium from sodium-roasted vanadium slag by fusion of leaching and extraction processes. Int J Miner Metall Mater 28, 974–980 (2021). https://doi.org/10.1007/s12613-020-2105-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-020-2105-1