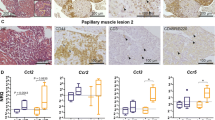
Abstract
Arrhythmogenic cardiomyopathy (AC) is an inherited disease characterized by progressive breakdown of heart muscle, myocardial tissue death, and fibrofatty replacement. In most cases of AC, the primary lesion occurs in one of the genes encoding desmosomal proteins, disruption of which increases membrane fragility at the intercalated disc. Disrupted, exposed desmosomal proteins also serve as epitopes that can trigger an autoimmune reaction. Damage to cell membranes and autoimmunity provoke myocardial inflammation, a key feature in early stages of the disease. In several preclinical models, targeting inflammation has been shown to blunt disease progression, but translation to the clinic has been sparse. Here we review current understanding of inflammatory pathways and how they interact with injured tissue and the immune system in AC. We further discuss the potential role of immunomodulatory therapies in AC.




Similar content being viewed by others
References
Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S, Pilichou K, Motta R, Aliberti C, Thiene G, McKenna WJ, Zorzi A, Iliceto S, Basso C, Perazzolo Marra M, Corrado D (2020) Arrhythmogenic right ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc. https://doi.org/10.1161/JAHA.119.014628
Corrado D, Basso C, Judge DP (2017) Arrhythmogenic cardiomyopathy. Circ Res 121:784–802. https://doi.org/10.1161/CIRCRESAHA.117.309345
Mazzanti A, Maragna R, Priori SG (2017) Genetic causes of sudden cardiac death in the young. Curr Opin Cardiol. https://doi.org/10.1097/HCO.0000000000000391
Mattesi G, Zorzi A, Corrado D, Cipriani A (2020) Natural history of arrhythmogenic cardiomyopathy. J Clin Med. https://doi.org/10.3390/jcm9030878
Corrado D, Link MS, Calkins H (2017) Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 376:61–72. https://doi.org/10.1056/NEJMra1509267
Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, Monteforte N, Memmi M, Gambelli P, Novelli V, Bloise R, Catalano O, Moro G, Tibollo V, Morini M, Bellazzi R, Napolitano C, Bagnardi V, Priori SG (2016) Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J Am Coll Cardiol 68:2540–2550. https://doi.org/10.1016/j.jacc.2016.09.951
Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W (2019) 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 16:e301–e372. https://doi.org/10.1016/j.hrthm.2019.05.007
Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, Lopez-Cuenca D, Sanchez-Munoz JJ, Oliva-Sandoval MJ, Gimeno JR (2015) Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 12:766–773. https://doi.org/10.1016/j.hrthm.2015.01.001
Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, Cooper LT, Wilber DJ, Marcus FI, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, Stevenson WG, McKenna WJ, Gautam S, Remick DG, Calkins H, Saffitz JE (2011) Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 4:743–752. https://doi.org/10.1161/CIRCEP.111.964890
Campian ME, Verberne HJ, Hardziyenka M, de Groot EAA, van Moerkerken AF, van Eck-Smit BLF, Tan HL (2010) Assessment of inflammation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging 37:2079–2085. https://doi.org/10.1007/s00259-010-1525-y
Protonotarios A, Wicks E, Ashworth M, Stephenson E, Guttmann O, Savvatis K, Sekhri N, Mohiddin SA, Syrris P, Menezes L, Elliott P (2019) Prevalence of 18F-fluorodeoxyglucose positron emission tomography abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol 284:99–104. https://doi.org/10.1016/j.ijcard.2018.10.083
Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, Agarwal PP, Arscott P, Dellefave-Castillo LM, Vorovich EE, Nutakki K, Wilsbacher LD, Priori SG, Jacoby DL, McNally EM, Helms AS (2020) Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 141:1872–1884. https://doi.org/10.1161/CIRCULATIONAHA.119.044934
Cristina B, Gaetano T, Domenico C, Annalisa A, Andrea N, Marialuisa V (1996) Arrhythmogenic right ventricular cardiomyopathy. Circulation 94:983–991. https://doi.org/10.1161/01.CIR.94.5.983
Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F (1997) Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol 30:1512–1520. https://doi.org/10.1016/S0735-1097(97)00332-X
Patrianakos AP, Protonotarios N, Nyktari E, Pagonidis K, Tsatsopoulou A, Parthenakis FI, Vardas PE (2012) Arrhythmogenic right ventricular cardiomyopathy/dysplasia and troponin release. Myocarditis or the “hot phase” of the disease? Int J Cardiol 157:e26-28. https://doi.org/10.1016/j.ijcard.2011.09.017
Valente M, Calabrese F, Thiene G, Angelini A, Basso C, Nava A, Rossi L (1998) In vivo evidence of apoptosis in arrhythmogenic right ventricular cardiomyopathy. Am J Pathol 152:479–484
Brodehl A, Belke DD, Garnett L, Martens K, Abdelfatah N, Rodriguez M, Diao C, Chen Y-X, Gordon PMK, Nygren A, Gerull B (2017) Transgenic mice overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS ONE. https://doi.org/10.1371/journal.pone.0174019
Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Alarcon N, Andersen P, Judge DP, Tung L, Saffitz JE (2019) Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 140:1491–1505. https://doi.org/10.1161/CIRCULATIONAHA.119.040676
Mavroidis M, Davos CH, Psarras S, Varela A, Athanasiadis CN, Katsimpoulas M, Kostavasili I, Maasch C, Vater A, van Tintelen JP, Capetanaki Y (2015) Complement system modulation as a target for treatment of arrhythmogenic cardiomyopathy. Basic Res Cardiol 110:27. https://doi.org/10.1007/s00395-015-0485-6
Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJB, de Bakker JMT, Tan HL, Valente M, Nava A, Wilde AAM, Moorman AFM, Thiene G, Bezzina CR (2009) Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med 206:1787–1802. https://doi.org/10.1084/jem.20090641
Lubos N, van der Gaag S, Gerçek M, Kant S, Leube RE, Krusche CA (2020) Inflammation shapes pathogenesis of murine arrhythmogenic cardiomyopathy. Basic Res Cardiol 115:42. https://doi.org/10.1007/s00395-020-0803-5
Priori SG, Remme CA (2020) Inherited conditions of arrhythmia: translating disease mechanisms to patient management. Cardiovasc Res 116:1539–1541. https://doi.org/10.1093/cvr/cvaa150
Gao S, Puthenvedu D, Lombardi R, Chen SN (2020) Established and emerging mechanisms in the pathogenesis of arrhythmogenic cardiomyopathy: a multifaceted disease. Int J Mol Sci. https://doi.org/10.3390/ijms21176320
Protonotarios A, Elliott PM (2020) Arrhythmogenic cardiomyopathy: a disease or merely a phenotype? Eur Cardiol 15:1–5. https://doi.org/10.15420/ecr.2019.05
Garrod D, Chidgey M (2008) Desmosome structure, composition and function. Biochim Biophys Acta 1778:572–587. https://doi.org/10.1016/j.bbamem.2007.07.014
Dubash AD, Green KJ (2011) Desmosomes. Curr Biol 21:R529-531. https://doi.org/10.1016/j.cub.2011.04.035
Austin KM, Trembley MA, Chandler SF, Sanders SP, Saffitz JE, Abrams DJ, Pu WT (2019) Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol 16:519–537. https://doi.org/10.1038/s41569-019-0200-7
Asimaki A (2020) Arrhythmogenic cardiomyopathy: an in-depth look at molecular mechanisms and clinical correlates. Trends Cardiovasc Med. https://doi.org/10.1016/j.tcm.2020.07.007
Carvajal-Huerta L (1998) Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol 39:418–421. https://doi.org/10.1016/s0190-9622(98)70317-2
McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355:2119–2124. https://doi.org/10.1016/S0140-6736(00)02379-5
Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP (2000) Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 9:2761–2766. https://doi.org/10.1093/hmg/9.18.2761
Que D, Yang P, Song X, Liu L (2015) Traditional vs. genetic pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Europace 17:1770–1776. https://doi.org/10.1093/europace/euv042
Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B, Arzamendi D, Flores J, Leung TK, Talajic M, Oliva A, Brugada R (2012) Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol 65:1077–1083. https://doi.org/10.1136/jclinpath-2012-201022
van Opbergen CJM, Noorman M, Pfenniger A, Copier JS, Vermij SH, Li Z, van der Nagel R, Zhang M, de Bakker JMT, Glass AM, Mohler PJ, Taffet SM, Vos MA, van Rijen HVM, Delmar M, van Veen TAB (2019) Plakophilin-2 haploinsufficiency causes calcium handling deficits and modulates the cardiac response towards stress. Int J Mol Sci. https://doi.org/10.3390/ijms20174076
Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP (2016) Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight. https://doi.org/10.1172/jci.insight.85923
Sebastian K, Bastian H, Magin TM, Krusche CA, Leube RE (2015) Desmoglein 2–dependent arrhythmogenic cardiomyopathy is caused by a loss of adhesive function. Circ Cardiovasc Genet 8:553–563. https://doi.org/10.1161/CIRCGENETICS.114.000974
Kraft L, Erdenesukh T, Sauter M, Tschöpe C, Klingel K (2019) Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res Cardiol 114:11. https://doi.org/10.1007/s00395-019-0719-0
He C, Carter AB (2015) The metabolic prospective and redox regulation of macrophage polarization. J Clin Cell Immunol. https://doi.org/10.4172/2155-9899.1000371
Ley K (2017) M1 means kill; M2 means heal. J Immunol 199:2191–2193. https://doi.org/10.4049/jimmunol.1701135
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang W, Zhong Y, Chen X, Chen Y, Sabri A, Liu S (2020) M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol 115:22. https://doi.org/10.1007/s00395-020-0781-7
Matsumori A, Kawai C (1980) Coxsackie virus B3 perimyocarditis in BALB/c mice: experimental model of chronic perimyocarditis in the right ventricle. J Pathol 131:97–106. https://doi.org/10.1002/path.1711310202
Bowles NE, Ni J, Marcus F, Towbin JA (2002) The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 39:892–895. https://doi.org/10.1016/s0735-1097(02)01688-1
Calabrese F, Basso C, Carturan E, Valente M, Thiene G (2006) Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovasc Pathol 15:11–17. https://doi.org/10.1016/j.carpath.2005.10.004
Chelko SP, Keceli G, Carpi A, Doti N, Agrimi J, Asimaki A, Beti CB, Miyamoto M, Amat-Codina N, Bedja D, Wei A-C, Murray B, Tichnell C, Kwon C, Calkins H, James CA, O’Rourke B, Halushka MK, Melloni E, Saffitz JE, Judge DP, Ruvo M, Kitsis RN, Andersen P, Lisa FD, Paolocci N (2021) Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abf0891
Psarras S, Mavroidis M, Sanoudou D, Davos CH, Xanthou G, Varela AE, Panoutsakopoulou V, Capetanaki Y (2012) Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J 33:1954–1963. https://doi.org/10.1093/eurheartj/ehr119
Meyer IS, Leuschner F (2018) The role of Wnt signaling in the healing myocardium: a focus on cell specificity. Basic Res Cardiol 113:44. https://doi.org/10.1007/s00395-018-0705-y
Rampazzo A, Calore M, van Hengel J, van Roy F (2014) Intercalated discs and arrhythmogenic cardiomyopathy. Circ Cardiovasc Genet 7:930–940. https://doi.org/10.1161/CIRCGENETICS.114.000645
Gemayel C, Pelliccia A, Thompson PD (2001) Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 38:1773–1781. https://doi.org/10.1016/s0735-1097(01)01654-0
Mu J, Zhang G, Xue D, Xi M, Qi J, Dong H (2017) Sudden cardiac death owing to arrhythmogenic right ventricular cardiomyopathy: two case reports and systematic literature review. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000008808
Priori SG, Mazzanti A (2020) Warning: not all carriers of pathogenic mutations in desmosomal genes should follow the same medical advices! Cardiovasc Res 116:1085–1088. https://doi.org/10.1093/cvr/cvaa049
Asimaki A, Kleber AG, Saffitz JE (2015) Pathogenesis of arrhythmogenic cardiomyopathy. Can J Cardiol 31:1313–1324. https://doi.org/10.1016/j.cjca.2015.04.012
Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D (2019) Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol 114:19. https://doi.org/10.1007/s00395-019-0722-5
Stadiotti I, Catto V, Casella M, Tondo C, Pompilio G, Sommariva E (2017) Arrhythmogenic cardiomyopathy: the guilty party in adipogenesis. J Cardiovasc Transl Res 10:446–454. https://doi.org/10.1007/s12265-017-9767-8
Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F (2018) Myocardial inflammation detected by cardiac MRI in arrhythmogenic right ventricular cardiomyopathy: a paediatric case series. Int J Cardiol 271:81–86. https://doi.org/10.1016/j.ijcard.2018.05.116
Chen L, Yang F, Chen X, Rao M, Zhang N-N, Chen K, Deng H, Song J-P, Hu S-S (2017) Comprehensive myocardial proteogenomics profiling reveals C/EBPα as the key factor in the lipid storage of ARVC. J Proteome Res 16:2863–2876. https://doi.org/10.1021/acs.jproteome.7b00165
Reis ES, Mastellos DC, Hajishengallis G, Lambris JD (2019) New insights into the immune functions of complement. Nat Rev Immunol 19:503–516. https://doi.org/10.1038/s41577-019-0168-x
Fry DE (2012) Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg 78:1–8
Mueller M, Herzog C, Larmann J, Schmitz M, Hilfiker-Kleiner D, Gessner JE, Theilmeier G (2013) The receptor for activated complement factor 5 (C5aR) conveys myocardial ischemic damage by mediating neutrophil transmigration. Immunobiology 218:1131–1138. https://doi.org/10.1016/j.imbio.2013.03.006
Chen L, Yi M, Song J, Hu S (2018) Complement system is highly activated and potentially acts as a biomarker for patients with ARVC. J Am Coll Cardiol 71:A745. https://doi.org/10.1016/S0735-1097(18)31286-5
Piriou N, Marteau L, Kyndt F, Serfaty JM, Toquet C, Le Gloan L, Warin-Fresse K, Guijarro D, Le Tourneau T, Conan E, Thollet A, Probst V, Trochu J-N (2020) Familial screening in case of acute myocarditis reveals inherited arrhythmogenic left ventricular cardiomyopathies. ESC Heart Fail 7:1520–1533. https://doi.org/10.1002/ehf2.12686
Thiene G, Basso C, Calabrese F, Angelini A, Valente M (2000) Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz 25:210–215. https://doi.org/10.1007/s000590050008
Calabrese F, Angelini A, Thiene G, Basso C, Nava A, Valente M (2000) No detection of enteroviral genome in the myocardium of patients with arrhythmogenic right ventricular cardiomyopathy. J Clin Pathol 53:382–387. https://doi.org/10.1136/jcp.53.5.382
Korkmaz S, Zitron E, Bangert A, Seyler C, Li S, Hegedüs P, Scherer D, Li J, Fink T, Schweizer PA, Giannitsis E, Karck M, Szabó G, Katus HA, Kaya Z (2013) Provocation of an autoimmune response to cardiac voltage-gated sodium channel NaV1.5 induces cardiac conduction defects in rats. J Am Coll Cardiol 62:340–349. https://doi.org/10.1016/j.jacc.2013.04.041
Li J, Maguy A, Duverger JE, Vigneault P, Comtois P, Shi Y, Tardif J-C, Thomas D, Nattel S (2014) Induced KCNQ1 autoimmunity accelerates cardiac repolarization in rabbits: potential significance in arrhythmogenesis and antiarrhythmic therapy. Heart Rhythm 11:2092–2100. https://doi.org/10.1016/j.hrthm.2014.07.040
Mavrogeni SI, Markousis-Mavrogenis G, Aggeli C, Tousoulis D, Kitas GD, Kolovou G, Iliodromitis EK, Sfikakis PP (2019) Arrhythmogenic inflammatory cardiomyopathy in autoimmune rheumatic diseases: a challenge for cardio-rheumatology. Diagnostics (Basel). https://doi.org/10.3390/diagnostics9040217
Caforio Alida L, Elisabetta Z, Annalisa V, Federica Re, Pasquale B, Francesco T, Gaetano T, Sabino I, Claudio T, McKenna William J (2008) Serum organ-specific anti-heart autoantibodies in arrhythmogenic right ventricular cardiomyopathy patients and relatives: evidence for autoimmune involvement in a genetically-determined myocarditis. Circulation. https://doi.org/10.1161/circ.118.suppl_18.S_948-c
Caforio Alida LP, Elisabetta Z, Federica Re, Pasquale B, Francesco B, Gaetano T, Sabino I, Petros S, McKenna William J (2011) Serum anti-intercalated disk autoantibodies in arrhythmogenic right ventricular cardiomyopathy are autoimmune markers associated with pathogenic desmosomal mutations. Circulation 124:A13404–A13404. https://doi.org/10.1161/circ.124.suppl_21.A13404
Caforio ALP, Re F, Avella A, Marcolongo R, Baratta P, Seguso M, Gallo N, Plebani M, Izquierdo-Bajo A, Cheng C-Y, Syrris P, Elliott PM, d’Amati G, Thiene G, Basso C, Gregori D, Iliceto S, Zachara E (2020) Evidence from family studies for autoimmunity in arrhythmogenic right ventricular cardiomyopathy: associations of circulating anti-heart and anti-intercalated disk autoantibodies with disease severity and family history. Circulation 141:1238–1248. https://doi.org/10.1161/CIRCULATIONAHA.119.043931
Peretto G, Barzaghi F, Cicalese MP, Resta CD, Slavich M, Benedetti S, Giangiobbe S, Rizzo S, Palmisano A, Esposito A, Cobelli FD, Gulletta S, Basso C, Casari G, Aiuti A, Bella PD, Sala S (2021) Immunosuppressive therapy in childhood-onset arrhythmogenic inflammatory cardiomyopathy. Pacing Clin Electrophysiol. https://doi.org/10.1111/pace.14153
Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, Payne AS (2016) Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353:179–184. https://doi.org/10.1126/science.aaf6756
Rosenblum MD, Gratz IK, Paw JS, Abbas AK (2012) Treating human autoimmunity: current practice and future prospects. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3003504
Zarak-Crnkovic M, Kania G, Jaźwa-Kusior A, Czepiel M, Wijnen WJ, Czyż J, Müller-Edenborn B, Vdovenko D, Lindner D, Gil-Cruz C, Bachmann M, Westermann D, Ludewig B, Distler O, Lüscher TF, Klingel K, Eriksson U, Błyszczuk P (2019) Heart non-specific effector CD4+ T cells protect from postinflammatory fibrosis and cardiac dysfunction in experimental autoimmune myocarditis. Basic Res Cardiol 115:6. https://doi.org/10.1007/s00395-019-0766-6
Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS (2014) Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm 30:389–394. https://doi.org/10.1016/j.joa.2013.12.008
Mesquita TRR, Zhang R, de Couto G, Valle J, Sanchez L, Rogers RG, Holm K, Liu W, Marbán E, Cingolani E (2020) Mechanisms of atrial fibrillation in aged rats with heart failure with preserved ejection fraction. Heart Rhythm 17:1025–1033. https://doi.org/10.1016/j.hrthm.2020.02.007
Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M (2017) Macrophages facilitate electrical conduction in the heart. Cell 169:510-522.e20. https://doi.org/10.1016/j.cell.2017.03.050
Bonny A, Lellouche N, Ditah I, Hidden-Lucet F, Yitemben MT, Granger B, Larrazet F, Frank R, Fontaine G (2010) C-reactive protein in arrhythmogenic right ventricular dysplasia/cardiomyopathy and relationship with ventricular tachycardia. Cardiol Res Pract. https://doi.org/10.4061/2010/919783
Panagopoulou P, Davos CH, Milner DJ, Varela E, Cameron J, Mann DL, Capetanaki Y (2008) Desmin mediates TNF-alpha-induced aggregate formation and intercalated disk reorganization in heart failure. J Cell Biol 181:761–775. https://doi.org/10.1083/jcb.200710049
Xue J, Yan X, Yang Y, Chen M, Wu L, Gou Z, Sun Z, Talabieke S, Zheng Y, Luo D (2019) Connexin 43 dephosphorylation contributes to arrhythmias and cardiomyocyte apoptosis in ischemia/reperfusion hearts. Basic Res Cardiol 114:40. https://doi.org/10.1007/s00395-019-0748-8
Lin C-Y, Chung F-P, Kuo L, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Tuan T-C, Chao T-F, Liao J-N, Chang T-Y, Yamada S, Te ALD, Huang T-C, Chen S-A (2019) Characteristics of recurrent ventricular tachyarrhythmia after catheter ablation in patients with arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol 30:582–592. https://doi.org/10.1111/jce.13853
Morel E, Manati AW, Nony P, Maucort-Boulch D, Bessière F, Cai X, de Besseyre Horts T, Janin A, Moreau A, Chevalier P (2018) Blockade of the renin-angiotensin-aldosterone system in patients with arrhythmogenic right ventricular dysplasia: a double-blind, multicenter, prospective, randomized, genotype-driven study (BRAVE study). Clin Cardiol 41:300–306. https://doi.org/10.1002/clc.22884
Bongianino R, Denegri M, Mazzanti A, Lodola F, Vollero A, Boncompagni S, Fasciano S, Rizzo G, Mangione D, Barbaro S, Di Fonso A, Napolitano C, Auricchio A, Protasi F, Priori SG (2017) Allele-specific silencing of mutant mrna rescues ultrastructural and arrhythmic phenotype in mice carriers of the R4496C mutation in the ryanodine receptor gene (RYR2). Circ Res 121:525–536. https://doi.org/10.1161/CIRCRESAHA.117.310882
Lodola F, Morone D, Denegri M, Bongianino R, Nakahama H, Rutigliano L, Gosetti R, Rizzo G, Vollero A, Buonocore M, Napolitano C, Condorelli G, Priori SG, Di Pasquale E (2016) Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis 7:e2393–e2393. https://doi.org/10.1038/cddis.2016.304
Karakikes I, Stillitano F, Nonnenmacher M, Tzimas C, Sanoudou D, Termglinchan V, Kong C-W, Rushing S, Hansen J, Ceholski D, Kolokathis F, Kremastinos D, Katoulis A, Ren L, Cohen N, Gho JMIH, Tsiapras D, Vink A, Wu JC, Asselbergs FW, Li RA, Hulot J-S, Kranias EG, Hajjar RJ (2015) Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun 6:6955. https://doi.org/10.1038/ncomms7955
Bezzerides VJ, Prondzynski M, Carrier L, Pu WT (2020) Gene therapy for inherited arrhythmias. Cardiovasc Res 116:1635–1650. https://doi.org/10.1093/cvr/cvaa107
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group (2017) Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Ji M, Lu Y, Zhao C, Gao W, He F, Zhang J, Zhao D, Qiu W, Wang Y (2016) C5a induces the synthesis of IL-6 and TNF-α in rat glomerular mesangial cells through mapk signaling pathways. PLoS ONE. https://doi.org/10.1371/journal.pone.0161867
Khameneh HJ, Ho AWS, Laudisi F, Derks H, Kandasamy M, Sivasankar B, Teng GG, Mortellaro A (2017) C5a Regulates IL-1β production and leukocyte recruitment in a murine model of monosodium urate crystal-induced peritonitis. Front Pharmacol 8:10. https://doi.org/10.3389/fphar.2017.00010
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther. https://doi.org/10.1038/sigtrans.2017.23
Sun S-C (2017) The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 17:545–558. https://doi.org/10.1038/nri.2017.52
Ferreira LMR, Muller YD, Bluestone JA, Tang Q (2019) Next-generation regulatory T cell therapy. Nat Rev Drug Discov 18:749–769. https://doi.org/10.1038/s41573-019-0041-4
Henning RJ (2018) Current status of stem cells in cardiac repair. Future Cardiol 14:181–192. https://doi.org/10.2217/fca-2017-0072
Marbán E (2018) A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat Biomed Eng 2:353–361. https://doi.org/10.1038/s41551-018-0216-z
Moore JB, Tang X-L, Zhao J, Fischer AG, Wu W-J, Uchida S, Gumpert AM, Stowers H, Wysoczynski M, Bolli R (2018) Epigenetically modified cardiac mesenchymal stromal cells limit myocardial fibrosis and promote functional recovery in a model of chronic ischemic cardiomyopathy. Basic Res Cardiol 114:3. https://doi.org/10.1007/s00395-018-0710-1
Polhemus DJ, Trivedi RK, Sharp TE, Li Z, Goodchild TT, Scarborough A, de Couto G, Marbán E, Lefer DJ (2019) Repeated cell transplantation and adjunct renal denervation in ischemic heart failure: exploring modalities for improving cell therapy efficacy. Basic Res Cardiol 114:9. https://doi.org/10.1007/s00395-019-0718-1
Wang L-T, Ting C-H, Yen M-L, Liu K-J, Sytwu H-K, Wu KK, Yen BL (2016) Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci 23:76. https://doi.org/10.1186/s12929-016-0289-5
Jin H, Sanberg PR, Henning RJ (2013) Human umbilical cord blood mononuclear cell-conditioned media inhibits hypoxic-induced apoptosis in human coronary artery endothelial cells and cardiac myocytes by activation of the survival protein Akt. Cell Transplant 22:1637–1650. https://doi.org/10.3727/096368912X661427
Jones SP, Trocha SD, Lefer DJ (2001) Cardioprotective actions of endogenous IL-10 are independent of iNOS. Am J Physiol Heart Circ Physiol 281:H48-52. https://doi.org/10.1152/ajpheart.2001.281.1.H48
Li J, Leschka S, Rutschow S, Schwimmbeck PL, Husmann L, Noutsias M, Westermann D, Poller W, Zeichhardt H, Klingel K, Tschope C, Schultheiss H-P, Pauschinger M (2007) Immunomodulation by interleukin-4 suppresses matrix metalloproteinases and improves cardiac function in murine myocarditis. Eur J Pharmacol 554:60–68. https://doi.org/10.1016/j.ejphar.2006.08.024
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E (2007) Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115:896–908. https://doi.org/10.1161/CIRCULATIONAHA.106.655209
Ibrahim AG-E, Cheng K, Marbán E (2014) Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2:606–619. https://doi.org/10.1016/j.stemcr.2014.04.006
Singh S, Chakravarty T, Chen P, Akhmerov A, Falk J, Friedman O, Zaman T, Ebinger JE, Gheorghiu M, Marbán L, Marbán E, Makkar RR (2020) Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res Cardiol 115:36. https://doi.org/10.1007/s00395-020-0795-1
de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marbán E (2017) Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 136:200–214. https://doi.org/10.1161/CIRCULATIONAHA.116.024590
de Couto G, Jaghatspanyan E, DeBerge M, Liu W, Luther K, Wang Y, Tang J, Thorp EB, Marbán E (2019) Mechanism of enhanced MerTK-dependent macrophage efferocytosis by extracellular vesicles. Arterioscler Thromb Vasc Biol 39:2082–2096. https://doi.org/10.1161/ATVBAHA.119.313115
Lin YN, Sanchez L, Zhang R, Ribeiro Mesquita TR, Li C, Ibrahim A, Cingolani E, Marban E (2020) Exosomes from engineered immortalized human heart cells decrease ventricular arrhythmias and attenuate fibrosis in mice with arrhythmogenic cardiomyopathy. Heart Rhythm 17(5):S1–S762
Takov K, He Z, Johnston HE, Timms JF, Guillot PV, Yellon DM, Davidson SM (2020) Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res Cardiol 115:26. https://doi.org/10.1007/s00395-020-0785-3
Zhang L, Zhu X-Y, Zhao Y, Eirin A, Liu L, Ferguson CM, Tang H, Lerman A, Lerman LO (2020) Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res Cardiol 115:16. https://doi.org/10.1007/s00395-019-0772-8
Chen P, Long B, Xu Y, Wu W, Zhang S (2018) Identification of crucial genes and pathways in human arrhythmogenic right ventricular cardiomyopathy by coexpression analysis. Front Physiol 9:1778. https://doi.org/10.3389/fphys.2018.01778
Lin Y, Liou Y-M, Chen J-Y, Chang K-C (2011) Sinus node dysfunction as an initial presentation of adult systemic lupus erythematosus. Lupus 20:1072–1075. https://doi.org/10.1177/0961203310396747
Gold J, Akazawa Y, Sun M, Hunter KS, Friedberg MK (2020) Relation between right ventricular wall stress, fibrosis, and function in right ventricular pressure loading. Am J Physiol Heart Circ Physiol 318:H366–H377. https://doi.org/10.1152/ajpheart.00343.2019
Acknowledgements
We thank Lisa Trahan for editorial assistance.
Funding
Y.N.L was supported by China Medical University (CMU107-N-05). General lab support was provided by grants from the National Institutes of Health (R01 HL124074, R01 HL135866 and R01 HL147570), the Peer-Reviewed Medical Research Program of the U.S. Department of Defense (PR150620), and the Cedars-Sinai Board of Governors. EM holds the Mark S. Siegel Family Foundation Distinguished Chair of the Cedars-Sinai Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.M. owns founder’s equity in Capricor Therapeutics.
Rights and permissions
About this article
Cite this article
Lin, YN., Ibrahim, A., Marbán, E. et al. Pathogenesis of arrhythmogenic cardiomyopathy: role of inflammation. Basic Res Cardiol 116, 39 (2021). https://doi.org/10.1007/s00395-021-00877-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-021-00877-5