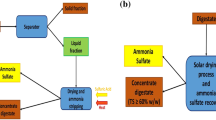
Abstract
This work investigates an innovative process to valorise agricultural digestate by the exploitation of solar energy. Digestate has been located in a lab-scale greenhouse to evaporate the liquid phase. Digestate vapours, rich in ammonia, are sent in a Drechsler trap, filled with 38% w/w sulfuric acid solution, through three solar air fans. A concentration of about 2 M of ammonium sulphate solution was recovered. The remaining dried solid phase, rich in phosphorous compounds, was evaluated as alternative to the commercial fertilizers (46% P2O5) in the growth of maize plants. Equal amount of P was applied to each pot (25 mg/kg soil). The plants were evaluated along the 8 weeks in a greenhouse monitoring the growth parameters and leaf SPAD index, micro-, macronutrients and non-essential heavy metals. The results evidenced that the dried solid phase of digestate can be used as an alternative source of P.
Graphic Abstract

Similar content being viewed by others
Statement of Novelty
This experimental work aimed to close the loop of the anaerobic digestion, showing the validity of the digestate for (i) the obtaining of a solution of ammonium sulfate, a commercial fertilizer, and (ii) its adoption as substitute of the conventional P-based fertilizer. These scopes were achieved through the exploitation of a totally clean energetical source, the solar one. In particular, the ammonium sulfate recovery was optimized by conduction of a preliminary stage of filtration to remove biggest solid particles, which was observed to be responsible of the ammonia adsorption, reducing the ammonium sulfate recovery. Then the dried digestate was tested as phosphorous nutrient to maize plants along two consecutive growth cycles, demonstrating very similar performances to the commercial fertilizers.
Introduction
In 2017 the European Union relaunched the efforts to limit to fossil fuels adoption in the economic activities in favor of renewable sources. Among them, agri-farm residues are the most abundant ones. Under the name of “agricultural residues”, two main typologies of wastes are embedded: primary residues are represented by solid vegetal residues and animal manure; secondary residues are the byproducts derived from one or more unit-operations for the production of an economic good (for instance, olive pomace from olive oil production) [1]. It was estimated that 395 million tonnes of dry matter (Tdm) of primary agricultural residues are annually produced in the EU countries but just 62 million Tdm are collectable as feedstock for the synthesis of bio-based materials, nutrients recovery and bioenergy applications [2]. Regarding the livestock sector, more than 1400 million tonnes of manure are estimated to be annually generated in the EU [3]. Animal manure is rich in nitrogen and phosphorous compounds, which are mainly lost (50–70%) to environment via NH3 volatilization, denitrification, leaching and run-off in pastures or during storage and/or following application of the animal manure to land [4]. The 2008/98 European Directive introduced the “waste hierarchy” concept, which promotes the production of bio-based materials and the recovery of nutrients from wastes as fundamental options in order to realize the transition from a linear economical model to the circular one [5]. When exploited, agri-farm residues are essentially adopted for bioenergy scope, in particular for the biogas production by anaerobic digestion (AD). Digestate is the main byproduct from AD, which can be adopted as soil improvers or fertilizer production in a circular economy optic. Digestate supplies stable carbon on fields thus increasing the carbon sink capability of soils and is rich in nitrogen (N), phosphorus (P), and potassium (K), important macro and micro- nutrients for intensive agriculture [6]. In particular, nitrogen is the most important and commonly lacking nutrient.
A fertile soil has the capacity to retain a reserve of essential nutrients for the crops, depending on the presence of clay particles and the organic matter’s composition of the soil. Only 2–3% of nitrogen compounds are in nitrate and ammonium forms, the ones which plants are able to assimilate [7]. The phosphorous compounds availability in the soil is even five folders lower than nitrogen ones [8]. Therefore, digestate valorization is able to provide a double beneficial scope: (i) closing the loop in the biogas AD process and (ii) recovering of nitrogen and phosphorous compounds, welcoming the indication of the “waste hierarchy” approach [9].
The application of agricultural digestate has been tested on the soil with the aim to increase the interaction of the soil–plant systems favoring the growth of the foliar area and weight of the vegetal and the distribution of photo assimilates between the different organs of the plants [10, 11]. The liquid and solid fractions of digestates contains macronutrients contain N and P compounds, respectively [12] and it was reported to be an alternative source of inorganic P fertilizers in several plant species such as amaranth and sorghum [13, 14], maize [13], plant ornamental species [15], ryegrass [16, 17], barley [18] and tomato [12]. But the direct digestate application on the soil has some drawbacks: bad odors, the presence of no stabilized compounds, of pathogens and, in some case, of heavy metals [19]. Consequently, the European Nitrate Directive, remark the need of an upgrade process for the digestate. Liquid fraction of digestate, rich in ammonia, is often sent to stripping process where ammonia is transferred from liquid to gas phase and converted in ammonium sulfate, a common N-based fertilizer, after the reaction with a solution of sulfuric acid [20,21,22]. The stripping process is characterized by high energy consumption to heat the digestate in the stripper till to 80 °C, and by high reagents (NaOH and H2SO4) consumption [23]. Instead, solid fraction rich in phosphorous can be exploited ad substitute of the common commercial P-based fertilizer [17].
The aim of this work had the ambitious to propose a model of circular economy focused on the use of the solar energy for a green exploitation of digestate, usually considered a by-product of the AD. In particular, the digestate drying and the ammonia recovery from the liquid phase of digestate was conducted by solar energy, with the avoiding of the consumption of electricity. An agriculture digestate, coming from a dry AD process, has been located in a transparent greenhouse, exposed to sunlight (Fig. 1).
After the drying process, the agricultural digestate was also tested as a P source for maize plants through pot experiments in a low P-containing silt loam soil comparing its performance to a commercial inorganic fertilizer (triple superphosphate, TSP). Two consecutive growth experiments were carried out using the same treated soils of the first one in order to evaluate the residual P fertilization effects of the different sources.
Materials and Methods
The recovery of Ammonium Sulfate Through the Drying of Digestate
Lab-Scale Solar Greenhouse Set-up
The ammonium sulfate recovery was tested on a dried agricultural digestate taken from a biogas plant treating a mixture of bovine manure, chicken manure and rice straw and operating at mesophilic conditions in dry conditions, ADDC (Agricultural Digestate Dry Condition) with a Total Solids (TS) content after AD of about 18% w/w.
The lab-scale greenhouse, adopted for the drying of the digestates, had the dimensions of 50 × 40 × 30 cm. In order to have temperatures comparable to the ones of the city of Sfax (where the BiogasMena project aims to realize the scale up of the process), the tests were conducted in summer. The lab-scale greenhouse was putted under the solar irradiation in a quiet zone of the garden of the Department of Biotechnology of the University of Verona (latitude 45°24′09″N, longitude 10°59′54″E). Verona city is located in the Po Valley (Northern Italy), having a continental climate with summer average temperatures of 29 °C and 23 °C in the maximum and minimal values, respectively. The rainfalls are distributed along all the year, with a major concentration in the hottest months [24].
10 L of the ADDC were fed in the greenhouse and were discharged when the TS concentration raised the value of 60% w/w. This is the minimal value of TS content required by Italian legislation for the preliminary drying process [25]. Before the feeding in the solar greenhouse, ADDC was processed with a preliminary filtration step (mesh size of 2 mm) in order to remove the fibers materials, which in our previous research work adsorbed ammonia molecules with a consequent reduction of the ammonium sulfate yield [6].
The digestate drying inside the greenhouse was facilitated by three air fans (Digiflex Solar Powered Cooling Fan) which had a diameter of 6 cm and a solar panel (5 × 6 cm). These solar fans had also the scope to carry the ammonia vapors from digestate into a Drechsler trap, a bottle provided with porous material filling to favor contact between sulfuric acid solution (38% w/w) and digestate’s vapors [22]. The greenhouse presented also a section shrinkage to increase speed of vapors towards the Drechsler trap. The porous material was constituted by borosilicate glass with porous dimensions in the range of 100–160 µm, which is usually used to gas transfer in a liquid phase. The amount of sulfuric acid solution in Drechsler trap was 0.4 L.
The Evaluation of the Test Performances
The performances of the test were evaluated essentially taking into account the following factors [6]:
-
i)
The concentration of ammonium sulfate solution recovered within the Drechsler trap from ADDC;
-
ii)
The yield of the ammonia recovery expressed as amount gone in the reaction with sulfuric acid and recovered as ammonium sulfate (ƞ), expressed as:
$$\eta \left(\%\right)=100 \,\frac{Amount\,of\,ammonia\, gone \,in\, reaction \,with \,sulfuric\, acid\, and \,recovered\, as \,ammonium\, sulfate \,(g)}{Amount\, of\, ammonia\, initially\, present \,in \,digestate\, (g)}$$(1)
where the numerator was calculated considering the ammonium sulfate concentration (M) in the Drechsler trap and the molar coefficients of Reaction 1.
The drying of ADDC was tested in duplicate in order to verify its repeatability and reduce the weather conditions’ influence.
Several parameters were considered for the characterization of ADDC at the beginning and at the end of the test: pH, total Chemical Oxygen Demand (COD), TS, Volatile Solids (VS), and the concentrations of total nitrogen compounds (TKN), ammonia and total phosphorus (TP). TS, VS, COD, TKN, ammonia and TP were determined using the standard methods described in the scientific literature [26]. To determine the ammonium sulfate content in the Drechsler trap solution, the back titration method has been adopted. In particular, this technique is based on dissociation of ammonia salts and the ammonia evaporation from solution by the addition of sodium hydroxide (Reaction 2) until to reach a pH of 11. At this condition all ammonia compounds were transferred in gaseous phase, which has been distilled by VELP UDK 159 distillation unit [27]. Then, 2–3 drops of phenolphthalein are added to the solution, which is then titrated with hydrochloric acid solution (0.1 N) (Reaction 3), until the red color is lost.
Exploitation of the Dried Digestate as Alternative to the Commercial P-Based Fertilizers
Description of the Pot Tests
Two sequential greenhouse pot experiments were carried out to evaluate the agricultural digestate (ADDC) as alternative P source to sustain the growth of maize seedlings comparing it to a commercial inorganic fertilizer (triple superphosphate, TSP, 46% P2O5). Taking into account the specific scope of Biogasmena project, which is focused on the implementation of a pilot anaerobic digester working at dry condition, ADDC were adopted for this specific part of the experimentation.
Considering the scope to evaluate the ADDC usage at the place of the P based commercial fertilizers a silt loam soil (sand 26%, slit 61.5% and clay 12.5%) with a low bioavailable P content (3.9 mg/kg) [28], was selected for this study. Its main physical–chemical characteristics were pH (H2O) value of 7.0, a cation exchange capacity (C.E.C) of 23.7 meq/100 g, a percentage of organic matter of 2.21. The experiment was carried out using 4.5-L pot containing 3.3 kg of soil treated with the same quantity of P equal to 82.5 mgP/pot (25 mgP/kg soil) corresponding to an agronomical P recommended dose of 96 kgP/ha for maize growth in a low P content soil [29]. In particular, 41.25 g/pot ADDC and 0.41 g/pot TSP with the 3.3 kg of soil were mixed in order to obtained ADDC-treated pot and TSP-treated ones. Five pots were set-up for ADDC treatment, five for the TSP treatment and five for negative control (C−, soil without addition of any P source). In addition, the quantity of N between pots treated with ADDC and those treated with TSP e C− were balanced with NH4NO3 to obtain the same dose of N per pot (240 mgN/pot, 72.72 mgN/kg soil, about, corresponding to 283 kgN/ha) [29]. All pots were fertilized with the same quantity of K (as KCl) equal to 42.18 mgK/pot (112.78 mgK/kg soil corresponding to 100 kgK/ha) [29] and 10 mL of a solution with the following composition: 0.05 mM ZnSO4, 0.02 mM CuSO4, 1 mM H3BO3, 0.001 mM (NH4)6Mo7O24, 0.04 mM Fe-EDTA and 2 mM CaSO4. Maize seeds (P0943 Hybrid, Pioneer Italia S.p.A.) were soaked in water for 24 h, germinated in the dark on wet filter paper for 48 h and then one seedling was transferred in each pot. Pot tests were located in a greenhouse (mean day temperature of 35 °C, a mean night temperature of 27 °C and a relative humidity of 45%) according to a randomized block scheme. Every week, the pots were re-organized in a new randomized block scheme in order to avoid differences due to different light conditions. In addition to sunlight, artificial light was provided to obtain a 16/8 h light/dark photoperiod.
Evaluation of the Pot Tests
During the growth experiment, the volumetric water content of each pot was regularly measured using the TDR 150 Soil Moisture Meter (FIELDSCOUT) and maintaining about 35% adding deionized water. Every 2 weeks pots were treated with 10 mL of a solution with the following composition [29]: 0.05 mM ZnSO4, 0.02 mM CuSO4, 1 mM H3BO3, 0.001 mM (NH4)6Mo7O24, 0.04 mM Fe-EDTA and 2 mM CaSO4. Starting from the third and second week for the first and second cycle respectively, leaf number, stem length and leaf SPAD (Soil–Plant Analysis Development) index were weakly measured. The average SPAD index of 7 measurements taken for each leave of the maize plants was recorded using the SPAD-502 (Konica Minolta). At the end of this first growth experiment (8 and 9 weeks for the first and second cycles respectively) a sample of soil was collected from each pot for the quantification of the available P and plants were harvested separating stem from root apparatus. The fresh weight (FW) was measured both for shoots and roots. The tissues were then washed 5 times with deionized water (18.2 MW·cm at 25 °C) and dried at 60 °C for 72 h, then weighted (dry weight, DW) and processed for the elemental analysis by ICP-MS. The same soil of each pot used for first growth experiment was collected and used for a second cycle of growth using the same experimental design. A new 2-day-old maize seedling was transferred in each pot. The growth was carried out in the same way and using the same conditions of the first cycle except for the duration (9 weeks) and the starting week of the collection of parameters (second week). Soil and plant tissue samples were collected and treated as previously described.
The concentration of available P in each soil samples was determined using 2 g of soil and following the method described by Olsen et al. [28].
The Multielemental analysis of plant tissues was performed as following. Dried plant tissues (about 10 mg) were mineralized in a 3-ml TFM microsampling insert (Milestone Srl) using 250 mL of ultrapure grade HNO3 (69%, Romil). The reaction was carried out at 180 °C for 20 min using a StartD (Milestone Srl) microwave digestor. Three inserts were placed in a TFM 100-mL vessel with 11 mL of Milli-Q water and 1 mL of ultrapure grade H2O2 (30%, Romil). The samples were then diluted to 2% HNO with ultra-pure grade water (18.2 MW·cm at 25 °C). The multielemental analysis of samples was performed using an Agilent 7500ce ICP-MS detection system (Agilent technologies). Calibration curves were obtained by diluting a custom-made multielement standard (Romil LTD). Measurement accuracy and matrix effect errors were checked using a standard reference material (NIST 1515 Apple leaves). Elements that were not measured accurately (more than ± 10% deviation from the certified value) were not further processed and reported in the result section.
Statistical Analysis
Statistical analyses were carried out through one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test using the GraphPad Prism 7 (GraphPad Software). Different letters indicate statistically significant differences (p < 0.05).
Results and Discussions
ADDC Characterization
Table 1 summarizes the physical and chemical characterization of the ADDC tested along the research work.
The pH value of ADDC was slightly basic at the beginning of the tests, as consequence of the high content of ammonia concentration (Table 1). ADDC had high VS/TS ratios, around 65–70%, with a lower value at the end of drying process as consequence of the anaerobic degradation of the organic matter. The amounts of carbon and nitrogen are fundamental to define the efficacy of a soil amendment. As reported by COD values in Table 1, the organic matter concentration in ADDC was of about 40 gCOD/L, lower than not filtered agricultural digestates, which show average COD contents around 45–50 g/L [6]. It was due as effect of the removal both of fibers and of all particles with diameter superior than 2 mm. Being organic matter, these materials contributes to the total COD increasing.
Regarding the nitrogen compounds, Kirchmann et al. [30] evidenced that, rather than the total concentration (TKN), is the balance between organic and mineral (ammonium) to influence the agronomic use of digestate. The higher is the share of ammonium, better is the efficiency of the digestate used as a N- based fertilizer [21]. In fact, ammonium is immediately available to be adsorbed and used by the plants; thus, high NH4+/TKN ratio is preferable because it reduces the volume needed for spreading on fields. Ammonium derives from the biological degradation of nitrogenous matter, mostly present in form of proteins and urea [31]. Typical protein rich substrates are manure and some typologies of food wastes [21, 32]. It allows to explain the high content of TKN and ammonia in ADDC.
Lastly, some considerations for phosphorous content in digestate. AD is known to not having a relevant ability to reduce the phosphorous compounds [17]. Consequently, it still contains a high level of phosphorus (either organic or inorganic phosphate) that, when directly discharged on soil, could be the cause of different environmental issues, firstly, eutrophication, which severely damages aquatic ecological systems. Table 1 seems to confirm that total phosphorous concentration remained high, even increasing after the solar drying process, as consequence of the liquid phase evaporation. Being mainly retained in the solid phase, phosphorous compounds in digestate could be converted in a precious resource after the drying process, closing the loop of the circular economy of the AD process. Phosphorus, in fact, is known to be a non-renewable plant nutrient and therefore essential for agriculture. Thus, digestate has been valorized as substitute of the conventional P-base fertilizer (Paragraph 3.3). By this way, the valorization of agricultural digestate would offer a green way of producing P based fertilizer. Currently phosphorous is unsustainably mined from phosphate rock, causing the depletion of this resource within the end of the century [33].
Ammonium Sulfate Recovery from Agricultural Digestate
The recovery of a solution rich in ammonium sulfate from different digestates was conducted in a previous our work [6], where ammonia adsorption phenomenon on solid particles was observed in agricultural digestate having a high content of TS. It represented a problem as it reduced the ammonia availability in the liquid phase, and so its recovery in the Drechsler trap. Along this experimental campaign, the process was optimized to increase the ammonium sulfate recovery even in presence of an agricultural digestate, rich in solids concentration.
Figure 2 [6] shows the effect of solar drying on ADDC (Fig. 2A), the formation of white ammonium sulfate crystals on a component of the Drechsler trap (Fig. 2B) and the ammonium sulfate solution recovered after a slow solvent evaporation at 60 °C (Fig. 2C). The ammonium sulfate crystals’ formation is probably due to a local increasing of the ammonium sulfate concentration in correspondence of the walls and of the other components of the Drechsler trap, which favored the precipitation of the salt.
ADDC at the end of the solar drying (A), the ammonium sulfate spontaneously crystalized within the trap (B) and ammonium sulfate recovered after solvent evaporation (C) (Battista and Bolzonella, [6])
Table 2 summarizes the concentrations of ammonium sulfate solutions in the Drechsler trap, the yield of ammonia gone in reaction with sulfuric acid (ƞ) and the time needed to reach the TS concentration of 60% w/w (t60).
ADDC reached the good ammonium sulfate concentration and the ammonia recovery yield (ƞ) respectively of 1.86 M and 65.02%. Our previous work [6] achieved lower performances with the same typology of digestate: of 1.04 M and a ƞ of 37.11% of ammonium sulfate concentration and anomia recovery yield, respectively. It can be explainable because fibers in ADDC are able to adsorb ammonia molecules impeding them the transferring from the liquid to the vapor phase [34].
Table 1 reports the characteristics of the dewatered ADDC too. Solar irradiation does not influence the VS/TS ratio, meaning that the aerobic degradation phenomena did not occurred and that substrates were well stabilized by AD. Also, the phosphorous compounds’ concentration kept constant after the drying process, while nitrogen compounds saw a decreasing, as consequence of ammonia evaporation. In fact, NH4+/TKN ratio showed a reduction of about 50–55%, demonstrating that organic nitrogen was almost not involved by the process and the remaining ammonia (1.01 g/kg) was probably absorbed by the finest fibers which was not filtered and remained in the ADDC.
Evaluation of the ADDC as an Alternative P Source for Maize Plants
After the drying operation in the solar greenhouse, ADDC was exploited as green substitute of the P commercial fertilizer (TSP) for the maize growth, conducted in two cycles. The second one was carried out in order to evaluate the residual effects of the two different P sources, reusing the soils of the first cycle.
First Growth Cycle
Plant growth parameters, such as leaf number and stem length and leaf SPAD index were weakly measured (Fig. 3) in order to compare the effects of ADDC and TSP. Additionally, at the end of the experiment (8 weeks) the FW and DW of shoots and roots were determined (Fig. 4). The treatment with ADDC significantly increased plant steam length, number of leaves (Fig. 3A, B) and shoot FW and DW (Fig. 4A, B) in comparison to not P-fertilized plants (C−), promoting a growth comparable to TSP-treated ones. A similar effect between an anaerobically digested orange waste and inorganic fertilizers on leaf numbers and shoot length of plants was reported for Chinese cabbage and ryegrass [35]. Considering the shoot FW and DW, our data are in line with the results reported in previous work where the comparison of the application of solid fraction of digestate and commonly used fertilizers investigated on different plant species [13, 14, 16, 17]. In particular, in pot experiments carried out with maize treated with the solid fractions of two digestates derived by different mixtures in the plant (dairy slurry, 57%, w/w and maize silage 43%, w/w and on energy crops only—87%, maize silage, 9% cereal whole plant silage, and 4% grass silage, w/w—respectively) was observed a production of dry matter measured similar to that obtained with NPK fertilizer [13]. Furthermore, the root FW weight of ADDC-treated plants showed no significant difference with the TSP-treated and untreated plants (C−) (Fig. 4C, D). In the case of root DW, highest values of biomass were recorded for the C− plants (Fig. 4D). The lowest shoot and the highest root DW observed for these plants grown in a low P available soil suggested that they exhibited the typical response to P deficiency based on the reduction in the shoot/root ratio brought about by a major inhibition in shoot growth rather than root [36]. Regarding plant leaf chlorophyll content, no significant differences were observed in SPAD index between the three nutritional conditions during the experiment (Fig. 3C) with the exception of data concerning the 5th week. These results suggested that the chlorophyll concentration in leaf is not affected by low availability of any macro- or micronutrients at least over the experiment [36, 37].
Leaf number (A), stem length (B) and SPAD index (C) of maize plants recorded during the first growth cycle (8 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
Shoot fresh (A) and dry (B) weight and root fresh (C) and dry (D) weight of maize plants at the end of the first growth cycle (8 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
Analysis of the plant P content determined by ICP-MS in shoot tissue at the end of the experiment (Fig. 5A), showed a similar pattern of the plant shoot biomass (Fig. 4A, B). Interestingly, the ADDC-treated plants accumulated P in shoot in similar quantity of TSP-treated ones (Fig. 5A). Similar results concerning total P (shoot + flowers) quantity was observed for pot experiment carried out with sunflower and marigold treated with steam-dried solid digestate and TSP as control [15]. In addition, no significant differences were reported in P uptake when plants of amaranth, maize and sorghum were fertilized with two solid digestates and inorganic NPK fertilizer [13]. At the end of the first cycle of growth, the available P concentration of soil was similar ADDC and TSP treatments (Fig. 5B). This confirms previous results reported for maize [13].
Total quantity of P accumulated in shoot (A) and (B) soil available P content determined at the end of the first growth cycle (8 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
We also investigated the total shoot content of other macronutrients, micronutrients and non-essential metals (Table 3). Plants fertilized with ADDC accumulated highest levels of K than TSP-treated and untreated plants (C−), whilst the levels of Ca and, in particular of Mg, were significant lower in shoot of ADDC-treated and C− plants (Table 3). Anyway, the contents of macronutrients in shoot (data not shown) were in all condition well above the optimal levels reported for shoot plants [38]. In alfalfa the fertilization with digestate increase the K content in plants in comparison to inorganic fertilizers [39]. A lower level of Mg content was recorded in aspen stem wood when seedlings were fertilized in pots with digestate in comparison to the treatment with sewage sludge and wood ash [40, 41]. In addition, both ADDC and TSP caused an increase in micronutrient total content particularly evident for B, Cu and Mn whilst for the levels of Mo were highest in response to ADDC supply (Table 3).
Analysing the non-essential metals, we observed that their concentration in shoot was higher in the unfertilized plants with the exception of Cd (Table 4). The plants treated with ADDC and TSP displayed similar concentration for all these metals that exhibited the highest level in P-fertilized plants (both with ADDC and TSP) and besides Na (Table 4).
Second Growth Cycle
The residual effect of the treatment of soil with different P sources was evaluated regrowing maize plants in the soil of the first experiment. Data show that in this cycle the plants fertilized with ADDC performed better than those treated with TSP as suggested by leaf number and stem length (Fig. 6A, B). On the other hand, as previously observed, any significant differences in leaf SPAD index between the three nutritional conditions was observed with the exception of data of the 6th week (Fig. 6C). Interestingly, in this second growth experiment the differences in shoot biomass between ADDC and TSP was more evident (Fig. 7A, B). Any significant difference was observed in weight of root apparatus (Fig. 7C, D). Taken together, these results show that in our experimental condition the residual fertilization capacity of ADDC is higher than TSP and able to sustain and enhanced growth of maize plants particularly considering shoot biomass accumulation.
Leaf number (A), stem length (B) and SPAD index (C) of maize plants recorded during the second growth cycle (9 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
Shoot fresh (A) and dry (B) weight and root fresh (C) and dry (D) weight of maize plants at the end of the second growth cycle (9 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
This consideration is also supported by the results concerning the P accumulation in shoot and the P available content of the soils measured at the end of the growth. In particular, in this cycle of experiment were more evident the differences between nutritional conditions in the shoot P content, with highest values for ADDC-treated plants (Fig. 8A). This trend agrees with that exhibited by the P available content of the soils (Fig. 8B). Similar considerations have been made in the case struvite [40,41,42]. In addition, Grigatti et al. [43] showed that the solid fraction of AD of maize had the highest potential P availability (Olsen-P) in comparison to inorganic P source (Ca(H2PO4)2*H2O), in line with our results.
Total quantity of P accumulated in shoot (A) and (B) and soil available P content determined at the end of the second growth cycle (9 weeks). Data are expressed as mean ± s.d. (n = 5 replicates; one-way ANOVA with Tukey’s post hoc test, p < 0.05, significant differences are indicated by different letters). C− negative control, no fertilization with P; TSP treatment with TSP; ADDC treatment with ADDC
In this second cycle of growth, the ADDC treatment improved the accumulation of nutrients in plant shoot. Regarding the content of other macronutrients at the end of the experiment (Table 5), the highest K concentration was determined in ADDC-treated plants. Interestingly, in this second cycle of growth were more evident the differences in K between plants treated with ADDC and TSP that exhibited similar level of the macronutrient to unfertilized ones (Tables 3 and 5). In addition, in this second experiment was less evident the differences in Mg as underlined by the similar level in the case of both P sources (Table 5). The ADDC fertilization displayed the highest values of several micronutrient as B, Cu, Mo, Ni and Zn not only in comparison with not-fertilized plants but also with TSP-treated ones (Table 5). It could be possible that the effect of organic matter applied to the soil with ADDC treatment is more evident in this second growth experiment, when the macronutrients availability (in particular for P) could become limiting. It is well known that organic matter affects soil physico-chemical properties that influence the availability of micronutrients as Zn, Cu, Fe, Mn, B and Mo [44]. Organic matter plays role in the distribution of micronutrients between soil colloids and solution. In particular, its high specific surface area, the cation exchange capacity and the presence of functional groups are involved in the formation of complexes with metals [44].
In this growth experiment, the shoot concentration of non-essential metals was in general higher in TSP- and ADDC-treated plants (Table 6). Only Cd and Na displayed the highest levels in response to the growth with ADDC. Anyway, the concentration of these metals was lower than those reported for tissues of maize plants grown in contaminated soils and those corresponding to limits of heavy metal in vegetables [45].
Conclusion
Agricultural digestate was adopted for agronomic scopes. Ammonia content was recovered from the liquid fraction of ADDC in order to obtain an ammonium sulfate solution, through an innovative operation which exploited the solar irradiation in a lab-scale greenhouse. A final solution of about 2 M was recovered when the process was optimized. The remaining dried solid phase of the agricultural digestate was tested as P-based fertilizer on the growth of maize plants. Its performances, compared to the conventional phosphorous fertilizers, led to similar plants’ length, weight and numbers of leaves. Also, the capacity to adsorb macro and micronutrients were similar, demonstrating the efficacy of agricultural digestate as soil improvers.
References
Bioenergy Europe: Biomass for energy: agricultural residues & energy crops. (2018).
Ronzon, T., Piotrowski, S.: Are primary agricultural residues promising feedstock for the European bioeconomy? Ind. Biotechnol. (2017). https://doi.org/10.1089/ind.2017.29078.tro
Flotats, X., Bonmatí, A., Palatsi, J., Foged, H.L.: Trends on manure processing in Europe. In: Raj, S. (ed.) Book of Proceedings, 2nd International Conference of WASTES: Solutions, Treatments and Opportunities, pp. 587–592. CVR Centro para a Valorizaçao de Residuos, Braga (2013)
Oenema, O., Tamminga, S.: Nitrogen in global animal production and management options for improving nitrogen use efficiency. Sci. China Ser. C. Life Sci. 48, 871–887 (2005)
Battista, F., Barampouti, E.M., Mai, S., Bolzonella, D., Malamis, D., Moustakas, K., Loizidou, M.: Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 131, 110007 (2020). https://doi.org/10.1016/j.rser.2020.110007
Battista, F., Bolzonella, D.: Exploitation of solar energy for ammonium sulfate recovery from anaerobic digestate of different origin. Waste Biomass Valor. 10(12), 3701–3709 (2019)
Fertilizers Europe: Balancing crop nutrition for healthy crop and fertile soils. www.fertilizerseurope.com. Access on 8th Feb 2021
EUROSTAT: eurostat.pdf. https://ec.europa.eu/eurostat/statisticsexplained/index.php/Farm_structure_statistics. (2018). Access on 8th Feb 2021
Barampouti, E.M., Mai, S., Malamis, D., Moustakas, K., Loizidou, M.: Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sustain. Energy Rev. 134, 110379 (2020). https://doi.org/10.1016/j.rser.2020.110379
Abubaker, J., Risberg, K., Pell, M.: Biogas residues as fertilisers—effects on wheat growth and soil microbial activities. Appl. Energy 99, 126–134 (2012)
Iocoli, G.A., Zabaloy, M.C., Pasdevicelli, G., Gómez, M.A.: Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci. Total Environ. 647, 11–19 (2019)
Ronga, D., et al.: Using digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 10, 138 (2020)
Bachmann, S., Uptmoor, R., Eichler-Löbermann, B.: Phosphorus distribution and availability in untreated and mechanically separated biogas digestates. Sci. Agric. 73, 9–17 (2016)
Hupfauf, S., Bachmann, S., Fernández-Delgado Juárez, M., Insam, H., Eichler-Löbermann, B.: Biogas digestates affect crop P uptake and soil microbial community composition. Sci. Total Environ. 542, 1144–1154 (2016)
Ehmann, A., Bach, I.M., Bilbao, J., Lewandowski, I., Müller, T.: Phosphates recycled from semi-liquid manure and digestate are suitable alternative fertilizers for ornamentals. Sci. Hortic. 243, 440–450 (2019)
Grigatti, M., et al.: Organic wastes as alternative sources of phosphorus for plant nutrition in a calcareous soil. Waste Manag. 93, 34–46 (2019)
Jimenez, J., Grigatti, M., Boanini, E., Patureau, D., Bernet, N.: The impact of biogas digestate typology on nutrient recovery for plant growth: accessibility indicators for first fertilization prediction. Waste Manag. 117, 18–31 (2020)
Maurer, C., Seiler-Petzold, J., Schulz, R., Müller, J.: Short-term nitrogen uptake of barley from differently processed biogas digestate in pot experiments. Energies 12, 696 (2019)
Wojnowska-Baryła, I., Bernat, K., Sartowska, S.: Biological stability of multi-component agri-food digestates and post-digestates. Waste Manag. 77, 140–146 (2018)
Battista, F., Frison, N., Bolzonella, D.: Energy and nutrients’ recovery in anaerobic digestion of agricultural biomass: an Italian perspective for future applications. Energies 12, 3287 (2019). https://doi.org/10.3390/en12173287
Vaneeckhaute, C., Lebuf, V., Michels, E., Belia, E., Vanrolleghem, P.A., Tack, F.M.G., Meers, E.: Nutrient recovery from digestate: systematic technology review and product classification. Waste Biomass Valor. 8, 21–40 (2017)
Stutzenstein, P., Bacher, M., Rosenau, T., Pfeifer, C.: Optimization of nutrient and carbon recovery from anaerobic digestate via hydrothermal carbonization and investigation of the influence of the process parameters. Waste Biomass Valor. 9, 1303–1318 (2018)
Bolzonella, D., Battista, F., Cavinato, C., Gottardo, M., Micolucci, F., Lyberatos, G., Pavan, P.: Recent developments in biohythane production from household food wastes: a review. Bioresour. Technol. 257, 311–319 (2018)
Average temperature along the year in Verona, Italy. http://en.wikipedia.org/wiki/Verone. Access on Jan 2021
http://www.camera.it/parlam/leggi/deleghe/10075dl.htm. Access on Jan 2021
APHA, AWWA, WEF: Standards Methods for the Examination of Water and Wastewater, 20th edn. United Book Press Inc., Baltimore (1998)
Teglia, C., Tremier, A., Martel, J.L.: Characterization of solid digestates: part 1, review of existing indicators to assess solid digestates agricultural use. Waste Biomass Valor. 2, 43–58 (2011)
Olsen, S., Cole, C., Watanabe, F., Dean, L.: Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular Nr 939. US Gov. Print. Office, Washington, D.C. (1954)
Perelli, M.: a cura di. Nutrire le piante. Trattato di scienza dei fertilizzanti. ARVAN, Mira (2009)
Kirchmann, H., Bernal, M.P.: Organic wastes treatment and C stabilization efficiency. Soil Biol. Biochem. 29, 1747–1753 (1997)
Battista, F., Fino, D., Erriquens, F., Mancini, G., Ruggeri, B.: Scaled-up experimental biogas production from two agro-food waste mixtures having high inhibitory compound concentrations. Renew. Energy 81, 71–77 (2015)
Risberg, K., Cederlund, H., Pell, M., Arthurson, V., Schnürer, A.: Comparative characterization of digestate versus pig slurry and cow manure—chemical composition and effects on soil microbial activity. Waste Manag. 61, 529–538 (2017)
Cordell, D., Drangert, J.-O., White, S.: The story of phosphorus: global food security and food for thought. Glob. Environ. Chang. 19(2), 292–305 (2009)
Zhao, Q.B., Ma, J., Zeb, I., Yu, L., Chen, S., Zheng, Y.M., Frear, C.: Ammonia recovery from anaerobic digester effluent through direct aeration. Chem. Eng. J. 279, 31–37 (2015)
Kaparaju, P., Rintala, J., Oikari, A.: Agricultural potential of anaerobically digested industrial orange waste with and without aerobic post-treatment. Environ. Technol. 33, 85–94 (2012)
Hawkesford, M., et al.: Chapter 6—functions of macronutrients. In: Marschners Mineral Nutrition of Higher Plants, pp. 135–189. Academic Press, Cambridge (2012). https://doi.org/10.1016/B978-0-12-384905-2.00006-6
Broadley, M., Brown, P., Cakmak, I., Rengel, Z., Zhao, F.: Chapter 7—function of nutrients: micronutrients. In: Marschner’s Mineral Nutrition of Higher Plants, pp. 191–248. Academic Press, Cambridge (2012). https://doi.org/10.1016/B978-0-12-384905-2.00007-8
Kirkby, E.: Chapter 1—introduction, definition and classification of nutrients. In: Marschner’s mineral nutrition of higher plants, pp. 3–5. Academic Press, Cambridge (2012). https://doi.org/10.1016/B978-0-12-384905-2.00001-7
Koszel, M., Lorencowicz, E.: Agricultural use of biogas digestate as a replacement fertilizers. Agric. Agric. Sci. Procedia. 7, 119–124 (2015)
Bertins, M., et al.: Impact of different fertilisers on elemental content in young hybrid aspen stem wood. Agron. Res. 18, 1154–1162 (2020)
Szymanska, M., Szara, E., Was, A., Sosulski, T., van Pruissen, G.W.P., Cornelissen, R.L.: Struvite—an innovative fertilizer from anaerobic digestate produced in a biorefinery. Energies 12, 296 (2019). https://doi.org/10.3390/en12020296
Szymanska, M., Sosulski, T., Bozetka, A., Dawidowicz, U., Was, A., Szara, E., Malak-Rawlikowska, A., Sulewski, P., van Pruissen, G.W.P., Cornelissen, R.L.: Evaluating the struvite recovered from anaerobic digestate in a farm bio-refinery as a slow-release fertiliser. Energies 13, 5342 (2020). https://doi.org/10.3390/en13205342
Grigatti, M., Boanini, E., Cavani, L., Ciavatta, C., Marzadori, C.: Phosphorus in digestate-based compost: chemical speciation and plant-availability. Waste Biomass Valor. 6, 481–493 (2015)
Dhaliwal, S.S., Naresh, R.K., Mandal, A., Singh, R., Dhaliwal, M.K.: Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: a review. Environ. Sustain. Indic. 1–2, 100007 (2019)
Adekiya, A.O., Oloruntoba, A.P., Ojeniyi, S.O., Ewulo, B.S.: Heavy metal composition of maize and tomato grown on contaminated soils. Open Agric. 3, 414–426 (2018)
Acknowledgements
This research is financed by Eranet Med Biogasmena (Project No. 72-026), which contemplates the optimization of technologies for biogas production and nutrients recovery from digestate in the MENA (Middle East and North Africa) regions.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have not conflict of interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the submitted work. I confirm that the manuscript has been read and approved by all named authors and there are no other persons who satisfied the criteria for authorship but are not listed. I further confirm that all of us have approved the order of authors in the manuscript. I declare that the paper has not previously submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Battista, F., Masala, C., Zamboni, A. et al. Valorisation of Agricultural Digestate for the Ammonium Sulfate Recovery and Soil Improvers Production. Waste Biomass Valor 12, 6903–6916 (2021). https://doi.org/10.1007/s12649-021-01486-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01486-y