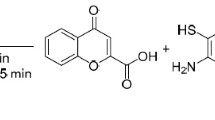
A method for the preparation of pyrimido[1,2-a]benzimidazoles containing a 2-hydroxybenzyl or (2-hydroxynaphthalen-1-yl)methyl group at position 3 was proposed based on the reaction of β-carbonyl-substituted 4H-chromenes and their benzo analogs with 2-aminobenzimidazole. In the case of chromenes substituted in the methylene fragment, 7,13a-dihydro-5H-benzo[5',6']chromeno-[3',2':5,6]pyrimido[1,2-a]benzimidazoles were isolated.


Similar content being viewed by others
References
(a) Begunov, R. S.; Ryzvanovich, G. A. Russ. Chem. Rev. 2013, 82, 77. [Usp. Khim. 2013, 82, 77.] (b) Goel, R.; Luxami, V.; Paul, K. RSC Adv. 2015, 5, 81608. b Jismy, B.; Akssira, M.; Knez, D.; Guillaumet, G.; Gobec, S.; Abarbri, M. New J. Chem. 2019, 43, 9961. c Fedotov, V. V.; Rusinov, V. L.; Ulomsky, E. N.; Mukhin, E. M.; Gorbunov, E. B.; Chupakhin, O. N. Chem. Heterocycl. Compd. 2021, 57, 383. [Khim. Geterotsikl. Soedin. 2021, 57, 383.]
Asobo, P. F.; Wahe, H.; Mbafor, J. T.; Nkengfack, A. E.; Fomum, Z. T.; Sopbue, E. F.; Döpp, D. J. Chem. Soc., Perkin Trans. 1 2001, 457.
Risley, V. A.; Henry, S.; Kosyrikhina, M. V.; Manzanares, M. R.; Payan, I.; Downer, C. D.; Hellmann, C. C.; Van Slambrouck, S.; Frolova, L. V. Chem. Heterocycl. Compd. 2014, 50, 185. [Khim. Geterotsikl. Soedin. 2014, 209.]
Shaaban, M. R.; Saleh, T. S.; Mayhoub, A. S.; Mansour, A.; Farag, A. M. Bioorg. Med. Chem. 2008, 16, 6344.
El-Shorbagi, A.-N. A.; Hussein, M. A. Pharma Chem. 2015, 7(4), 190.
Zhang, Z.-T.; Qiu, L.; Xue, D.; Wu, J.; Xu, F.-F. J. Comb. Chem. 2010, 12, 225.
Rawat, M.; Rawat, D. S. Tetrahedron Lett. 2018, 59, 2341.
Zanatta, N.; Amaral, S. S.; Esteves-Souza, A.; Echevarria, A.; Brondani, P. B.; Flores, D. C.; Bonacorso, H. G.; Flores, A. F. C.; Martins, M. A. P. Synthesis 2006, 2305.
Kreutzberger, A.; Leger, M. Arch. Pharm. (Weinheim, Ger.) 1982, 315, 438.
(a) Ryabukhin, S. V.; Plaskon, A. S.; Volochnyuk, D. M.; Pipko, S. E.; Tolmachev, A. A. Heterocycles 2008, 75, 583. (b) Badran, A.-S.; El-Gohary, N. M.; Ibrahim, M. A.; Hashiem, S. H. J. Heterocycl. Chem. 2020, 57, 2570.
(a) Kreutzberger, A.; Leger, M. J. Heterocycl. Chem. 1981, 18, 1587. (b) Saijo, R.; Watanabe, G.; Kurihara, K.; Kawase, M. Heterocycles 2014, 89, 2334.
Bouillon, J.-P.; Janousek, Z.; Viehe, H. G.; Tinant, B.; Declercq, J.-P. J. Chem. Soc., Perkin Trans. 1 1995, 2907.
Krasovsky, A. L.; Hartulyari, A. S.; Nenajdenko, V. G.; Balenkova, E. S. Synthesis 2002, 133.
Kawase, M.; Hirabayashi, M.; Saito, S.; Yamamoto, K. Tetrahedron Lett. 1999, 40, 2541.
(a) Belyaev, D. V.; Chizhov, D. L.; Kodess, M. I.; Ezhikova, M. A.; Rusinov, G. L.; Charushin, V. N. Mendeleev Commun. 2019, 29, 249. (b) Goryaeva, M. V.; Burgart, Ya. V.; Saloutin, V. I.; Chupakhin, O. N. Chem. Heterocycl. Compd. 2012, 48, 372. [Khim. Geterotsikl. Soedin. 2012, 395.] (с) Goryaeva, M. V.; Burgart, Ya, V.; Saloutin, V. I. Russ. J. Org. Chem. 2010, 46, 432. [Zh. Org. Khim. 2010, 46, 437.]
Tseng, S.-S.; Epstein, J. W.; Brabander, H. J.; Francisco, G. J. Heterocycl. Chem. 1987, 24, 837.
Osipov, D. V.; Osyanin, V. A.; Klimochkin, Yu. N. Targets Heterocycl. Syst. 2018, 22, 436.
(a) Lukashenko, A. V.; Osyanin, V. A.; Osipov, D. V.; Klimochkin, Yu. N. J. Org. Chem. 2017, 82, 1517. (b) Lukashenko, A. V.; Osyanin, V. A.; Osipov, D. V.; Klimochkin, Yu. N. Chem. Heterocycl. Compd. 2016, 52, 711. [Khim. Geterotsikl. Soedin. 2016, 52, 711.]
Shakibaei, G. I.; Mirzaei, P.; Bazgir, A. Appl. Catal., A 2007, 325, 188.
CrysAlisPro, version 1.171.38.41; Rigaku Oxford Diffraction, 2015. https://www.rigaku.com/en/products/smc/crysalis.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
This work was financially supported by the Russian Foundation for Basic Research (contract No. 18-33- 20249).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(5), 588–593
Supplementary Information
ESM 1
(PDF 449 kb)
Rights and permissions
About this article
Cite this article
Osyanin, V.А., Osipov, D.V., Korzhenko, K.S. et al. 4H-Chromenes as 1,3-bielectrophiles in the reaction with 2-aminobenzimidazole: synthesis of pyrimido[1,2-a]benzimidazoles. Chem Heterocycl Comp 57, 588–593 (2021). https://doi.org/10.1007/s10593-021-02947-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02947-x